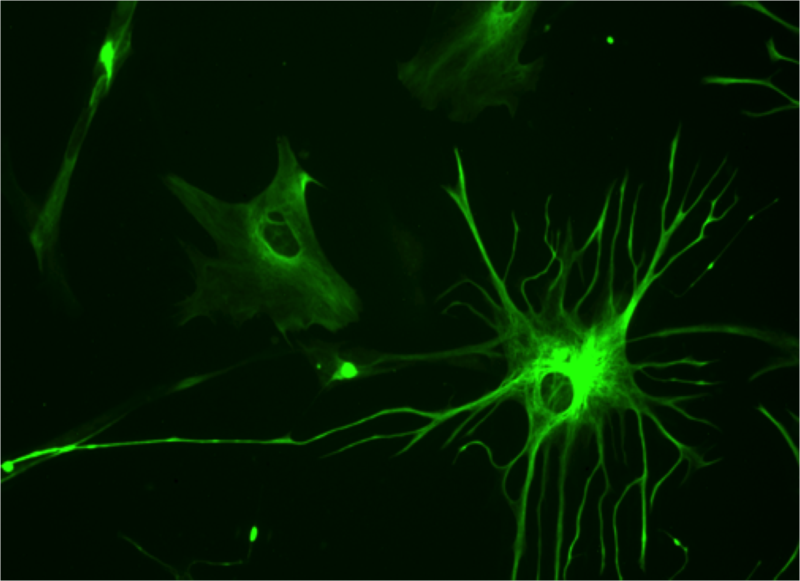
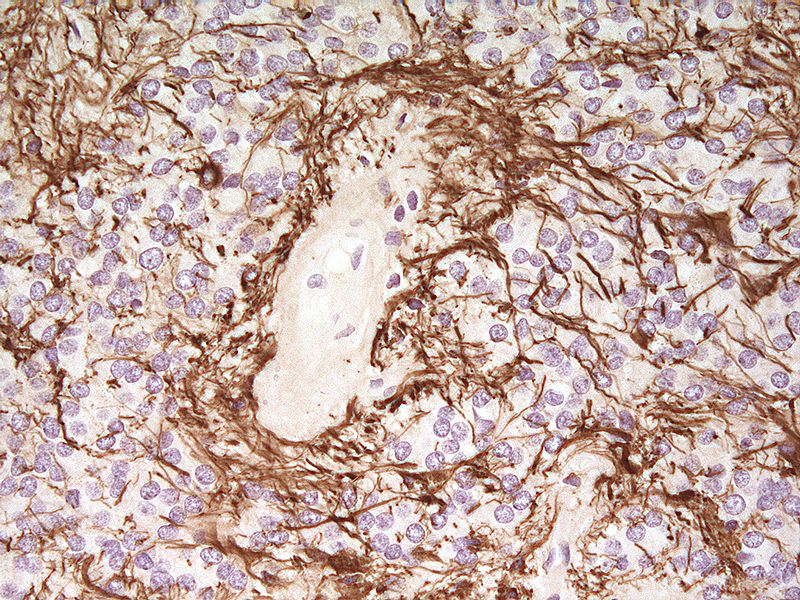
Reactive Gliosis
The reactive gliosis, sometimes called Astrogliosis or simply Gliosis is refered to a physiological process appearing as a result of damage to the CNS (eg. traumatic brain Injury “TBI”, ischemia, neurodegenerative diseases). Reactive gliosis is as response by glial cells, mainly astrocytes, reacting to an injury and can be reviewed as a healing process. After a CNS injury, the astrocytes around the lesion respond to injury and undergo a typical change of hyperthrophy. These reactive astrocytes are gradually intergrated and form a physical barrier, commonly refered to as glial scar.
Contents
Glial Cells
|
The brain tissue contain two categories of cells, nervous cells and glial cells or, neuroglia. Glial cells are about 10-50 time more numerous than nerve cells and are involved in processes like:
- Supply nutrients and oxygen
- Regulation of homeostasis
- Neurotransmission
- Structural support
- Insulation (Myelinating the axons)
- Destroy pathogens and remove dead neurons and metabolites
Following glial cells are the main cells involved in reactive gliosis:
Astrocytes
This is the most abundant glial cell type in the CNS. There two different types of astrocytes according to their location and morphology: protoplasmic astrocytes with characteristic branched processes, which are mainly found in the grey matter, and fibrous astrocytes found mainly in the white matter with fiber-like processes. Astrocytes act as the main regulator of the CNS to sustain homeostatic environment, which is a necessity for regular neurological cell activity. This includes the ionic concentration regulation and secretion and degeneration of neurotransmitters. Astrocytes are connected to each other through gap junctions forming a network called astrocyte syncytium which enables them to directly communicate which each other. They also interact as a bidirectional bridge between the neurons and bloodstream. One of the functions of this communication is energy supply of the neurons, astrocytes is therefore highly related with the neuronal metabolism. Glucose and it´s metabolites are transmitted from the blood to the neurons and neighbouring through glucose transporters. Another function related to its close situation with blood vessels it the aid of the blood brain barrier. Astrocytes take part in numerous actions of the brain and the view and description of its functions have changed over time along with knowledge and research to a cell type with much more and advanced functions than earlier believed (Andersson, 2011; Widestrand, 2008).
Microglia
Microglia is a local type of macrophage in the CNS with its main function directed to the immune defense. It constitutes approximately 5-20% of the total glial cell count and is most abundant in the grey matter. During normal conditions the microglia is in a "resting stage" where it have a ramified morphology with small processes, a unique look of the macrophage family. The microglia are extremely sensitive to any disturbance of the internal environment that can indicate on a potential danger to the CNS. In case of such alteration of homeostasis, microglia will be activated. The cell will change in shape, gene expression and its function to counter the potential danger, this is what is called the “microglial activation” (Kettenmann et al, 2011).
Oligodendrocytes
Possessing few processes they are specializes in myelination of neurons’ axons. Their processes extend toward axons and wrap around them. Then they produce myelin to create the myelin sheet that accelerate neuronal transmission. One oligodendrocyte can myelinate several axons and are more abundant in the white matter.
NG2-Expressing Cells
Discovered a few years ago and today a fairly new accepted member of the glial cells gives the explanation to that their function in the brain is not yet fully understood. Their name derives from the ability to express chondroitin sulfate proteoglycan (NG2). They have several functions including being a precursor of other glial cells (Andersson, 2011).
Damage of the nervous system
CNS damage is a two step process starting with the primary injury and a later a secondary damage phase involving a cascade of auto destructive injury (Okada et al, 2009).
Primary damage to the CNS can be of different origin, it can for example occur from:
- External trauma: TBI (traumatic brain injury), SCI (spinal cord injury)
- Ischemia: Restriction in blood supply which lead to shortage of nutrients and oxygen (e.g. Stroke)
- Neurodegenerative diseases (e.g. Alzheimer´s)
In general the primary damage leads to cell death, swelling, release of toxic molecules (e.g. free radicals, nitric oxide, glutamate). Also one of the most significant threats is the penetration of the blood brain barrier (BBB), which will disrupt the normally pathogen free inner CNS environment. All of this factors will lead to the secondary damage phase which will result to a expanded area of damage due to enhanced effects and reactions of the primary damage. If not stopped this may lead to a even more life threatening chain reaction of cell death. This is why the brain has to activate a pathway to stop these processes and try to keep the integrity of the CNS.
Process
|
The primary damage of CNS triggers the glial cells by the secretion of activating factors including inflammatory cytokines, growth factors and extracellular matrix molecules. This activation factors will induce the change (activation) of the glial cells into responding to the primary damage, this is what in general is referred to as the reactive gliosis. The main result of the reactive gliosis will be the formation of the glial scar. The glial scar is a meshwork of glial cells (essentially astrocytes and microglial cells) made by interwoven cell processes.
The activity of reactive gliosis can be measured through the ratio change of intermediate filaments in astrocytes due to their change of activity. Intermediate filaments are one of the three major components in the cytoskeleton (microtubules, actin filaments and intermediate filaments) of cells. Over 50 different intermediate filament proteins have been identified. Astrocytes express 4 different types of IF proteins, in which the most important in the activity measurement is the GFAP (glial-fibrillary acidic protein) and Vimentin which will show a distinct measureable increase when astrocytes becomes reactive, in other words due to reactive gliosis (Widestrand, 2008).
Microglia
Due to microglia being extremely sensitive to changes in the micro environment (e.g. primary damage) they will respond by self activation. After their activation the microglia will produce factors that will activate astrocytes. The activation of Astrocytes is a key element of reactive gliosis since astrocytes play an important structural role in the gliosis. Microglial cells are also known to enhance further immune response and inflammation reaction. Secreted molecules are for example cytokines and growth factors. Those factors are responsible for further response by cell accumulation and inflammation reaction (Andersson, 2011; Widestrand, 2008).
Astrocytes
Reactive astrocytes serve to carry out the main cellular response during reactive gliosis. Characteristic features of this response include hypertrophy, proliferation and enhanced intermediate filament expression (GFAP, Vimentin). Astrocytes will migrate toward the place of injury, and by their hypertrophied state they will fill the space where neurons die and by this restore the structural integrity and protective barrier. At the place of injury they will also weave their processes with other glial cells, this agglutination form the glial scar (Andersson, 2011; Widestrand, 2008).
Effects of reactive gliosis
Reactive gliosis and the forming of the glial scar is the imidiate response to protect the CNS from extensive neuronal damage. But this response might also alter the future recovery process in a negative way that even do significally more damage than the primary damage. If there is a damage in a certain area of the brain there will be very little regeneration of the neurons if any at all. This generally leads to a permanent impairment of neuronal function. The aim of to-days research in the subject of reactive gliosis is to get a better understanding of the whole mechanism and by these means be able to prevent the negative side effects of the gliosis. Further understanding about what positive and negative effects follow a reactive gliosis will be described in this section
Positive and negative effects following reactive gliosis
When damage occurs in the CNS the brain will protect itself by isolating the area affected of the damage by producing a barrier, the glial scar, this is mainly in order to reestablish the blood brain barrier. Damage to the CNS will follow a two-way-process, the primary injury followed by an auto-destructive injury cascade. The auto-destructive cascade have to be stopped in order to isolate the extent of damage in such a sensitive system as the CNS. The forming of the glial scar is essential in order to protect the brain from further damage. The hypertrophied astrocytes form a dense network to prevent spreading of pathogens and the auto-destructive injury cascade. The astrocytes have a vital role in the elimination of free radicals, especially during an injury when a large amount of free radicals are released from dying cells (Andersson, 2011; Widestrand, 2008).
The positive effects can mainly be found in the acute phase of an injury where as the period following is marked by negative side effects of the gliosis. In most cases this contra productive side effects can be of much importance due to it outweighing the positive (protective effects) of the gliosis. One primary side effects is that glial scar will inhibit long time neuronal regrowth in the affected are, for example by certain myelin inhibiting proteins released in the leisure area, which inhibits axonal regrowth. The glial cells in the affected area will keep secreting this proteins as long as there is an active process of scar formation. This will eventually lead to a permanent impairment of neuronal function (Goldshmit et al, 2004).
Examples of this myelin inhibiting proteins are Nogo, Myelin-associated glycoprotein (MAG) and Oligodendrocyte-myelin glycoprotein (OMgp). All of these proteins are thought to inhibit the same mechanism of the neurons which eventually by certain interactions with axons lead to their inhibition of axonal outgrowth. Even when blocking these proteins after an injury to the CNS the neuronal recovery will not be significantly improved (Goldshmit et al, 2004).
The negative effects can commonly be derived from the glial scar. The astrocytes involved in this physical barrier produce GFAP which in turn helps in the forming of the dense network of processes connecting one astrocyte to another. This morphological changes are accompanied by several chemical factors like cytokines and cell adhesion substances. Some of this molecules have an inhibitory effect on the neuronal regrowth e.g. Chondroitine sulphate proteoglycan (GSPG) and collagen IV.
As mentioned earlier the negative effects of the reactive gliosis could sometimes be so substantial that the aftermath of the reactive gliosis could be much worse than the initial damage. Inhibition of the neuronal regeneration could lead to e.g. permanent paralysis from damages to the spinal cord whereas the fibers should be able to regenerate by them self, but contradictory are inhibited by the chemical and physical barrier of the remaining glial scar(3). Neurodegenerative diseases such as Alzheimer's disease, have a greater impact in the combination with gliosis. When the disease damage the brain tissue, even in a very small scale, the final damage will be substantially more extensive (Okada et al, 2009).
Benefits of future research
CNS related complications constitutes one of the highest cause of death in the western world, not to mention all other daily life problems people affected by CNS injuries has to live with. The research about reactive gliosis is one of the landmarks in helping to understand this issue and hopefully also lead to improvements in protection and treatment of CNS damage.
One of the main aspects in this topic is that even if the primary damage to the brain (TBI, SCI, Ischemia, Neurodegenerative diseases) might cause substantial harm, the secondary process involving reactive gliosis is much more harmful in a longer perspective. Learning to control this process might lead to that in the future we can for example:
- Recover from CNS damage that we today don´t expect any remarkable or no improvements in
- Protection from the negative effects of the secondary damage process (reactive gliosis)
The knowledge and treatment methods in this field is improving each day, but much is yet to be discovered.
References
Other references
6. Andersson H. (2011): Reactive gliosis in the injured brain.(thesis) - http://hdl.handle.net/2077/23937
7. Widestrand Å. (2008): The effect of astrocytes and reactive gliosis on neurogenesis and astrogenesis in mouse.(thesis) - http://hdl.handle.net/2077/18670
Pictures