Analysis of heart rate variability
Contents
-
Analysis of heart rate variability
- Introduction
- Effects of respiration on heart rate variability
- Effect of disease on Heart Rate Variability
- Effect of age on Heart Rate Variability:
- Effect of gender on Heart Rate Variability:
- Effect of ethnic differences on Heart Rate Variabilty:
- Effect of alcohol on Heart Rate Variabilty:
- Conclusion
- Bibliography:
Introduction
As stated by (Almeida-Santos and Sousa, 2015), Heart rate variability (HRV) is a parameter that measures time variations of sinus rhythm, reflecting regulatory effects of the nervous system and humoral factors on the sinoatrial node (Almeida-Santos and Sousa, 2015). Measuring HRV is a dependable way to evaluate differences in the autonomic nervous system (Peng et al., 2015). When we discuss heart rate variability, we must include the standard deviation of normal-to-normal RR intervals (SDNN) and also the root mean square of successive differences (RMSSD). The (SDNN) describes both the sympathetic and vagal activity, while the (RMSSD) describes mostly the sympathetic activity (Yi et al., 2014). There are numerous methods of measuring heart rate variability. One of the most common methods is using electrocardiography (ECG). 24 hour ECG recordings are completed when the test subject goes about their regular life. These recordings are very useful for analysing the risk of pathological problems and for assessing autonomic dysfunction.
Effects of respiration on heart rate variability
Respiration has an effect on the heart rate variability in two ways:
Firstly, the baroreceptors or stretch receptor reflexes sense the changes of arterial pressure and forward the information to the autonomic nervous system. They have an inhibitory effect on the sympathetic output and a stimulating effect on motorneurons of the vagal system. (Bernston et al, 1997). These receptors work in phases in a short space of time and under maximal heart rate variability frequency ranges(Kezdi and Geller, 1968). It is the respiratory effect of both the central and peripheral centres that regulates the baroreceptor reflexes. The respiratory centres also regulate the activities of both the spinal sympathetic and vagal motor neurons which vary with each breath. (Eckberg et al, 1988).
Secondly is via the direct connection between the sinoatrial node located in the wall of the right atrium of the heart and the respiratory frequency rhythms of the autonomic nerves.The respiratory frequency rhythms are translated into changes in discharge frequency of the SA node, thus RSA (Respiratory sinus arrhythmia) differs with breathing rate (Bernston et al., 1997). SA can be considerably greater during slow breathing than during fast breathing (Witte and Rother, 1992).
Symapathovagal Balance and Heart Rate Variability:
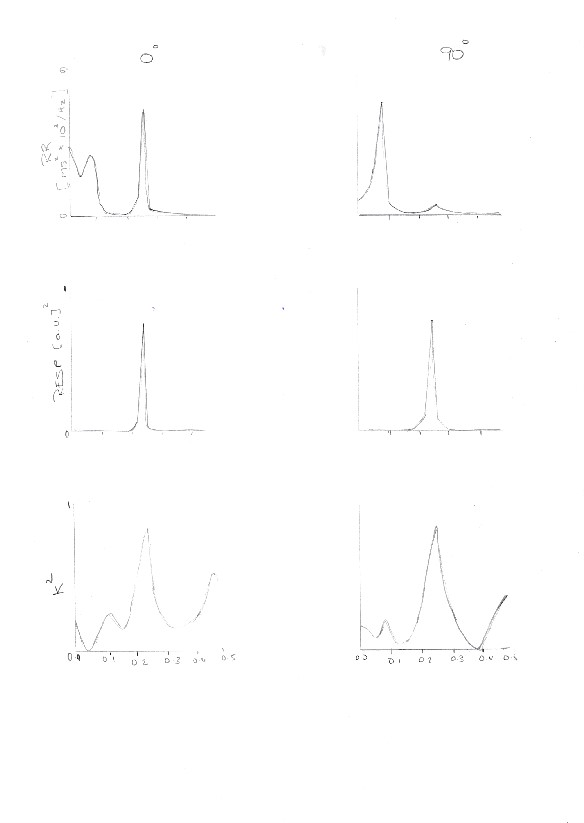
An experiment which proves the connection between respiration and heart rate variability is “The Power Spectrum Analysis of Heart Rate Variability to assess the changes in symapathovagal Balance during graded orthostatic tilt”. (Montano et al.,1994). The original hypothesis behind the experiment was that the connection between the sympathetic and vagal regulations of the sinus node in the heart are complementary, i.e increased activity of the sympathetic system, results in the decreased activity of the vagus system. (Schwartz et al., 1973) They followed 22 healthy volunteers and subjected them to passive head tilts at a series of selectively chosen angles: 15°, 30°, 45°, 60°, 90°. These passive head tilts were carried out after a period of rest. They calculated the time and frequency indexes of 13RR interval variability (See figure 1) (Montano et al., 1994) They measured respiratory activity using a nasal thermistor.
The 2 most important oscillatory factors were the High Frequency (HF), which is concurrent with respiration, (Mayer S, Koepchen HP) and Low Frequency (LF) which relates to the slow waves of arterial pressure. (Malliani et al., 1991).The respiratory cycle signal was measured once every cardiac cycle in correspondence with the R wave, thus obtaining a repirogram synchronised with a tachogram.
They concluded that an upright position is responsible for slight tachycardia (common heart disorder), the neural regulation of SA node in the heart is affected by the sympathetic excitation and vagal withdrawal which accompany an upright position (Rushmer, 1962).
The effect of smoking on the heart rate variability:
In a study conducted in Japan by Junichi Minami, Toshikiko Ishimitsu, Hiroaki Matsuoka, the effect of 1 week smoking cessation on heart variability among other parameters was investigated. The test subjects were habitual male cigarette smokers,smoking more than 10 cigarettes daily (Range 10-80). Their results showed a clear correlation between cigarette smoking and heart variability. They found that not just the daytime HRV but also the nighttime HRV was significantly lower in the nonsmoking period than the smoking period. The number of total heart beats over a 24 hour period was 10,471 +/ - 188 beats less in the non smoking period. (Minami et al., 2018) They also recorded the average values of PNN50 (defined as the mean number of times an hour in which the change in successive normal sinus (NN) intervals exceeds 50 ms 1) (Mietus, 2002).
This measurement showed that there was also a correlation between smoking cessation and decreased PNN50 over a 24 hour period.However the results also showed that PNN50 was higher during the non-smoking period 15.6+/-2.1% , than during the smoking period 10.0+/-1.6%,indicating that no smoking raised the parasympathetic nerve activity over a 24 hour period (Minami et al., 2018).
|
Effect of disease on Heart Rate Variability
The effects that a disease may have on heart rate variability, or the irregularities of heart rate variability which informs us of an underlining physiological problem has been demonstrated by many different experiments and has proved useful in aiding diagnoses and treatment of diseases. Two diseases that affect heart rate variability are: Gastric Cancer (GC), and Diabetes mellitus (type one) ( Kessler and Karimov, 2014).
Effect of Gastric Cancer on heart rate variability:
Gastric Cancer is a frequent type of gastrointestinal cancer that has an extremely high mortality rate among common cancers (Park et al., 2014). The study investigated changes of SDNN and RMSSD on patients with Gastric Cancer in order to scrutinize the predicting role of HRV in relation to the disease. The result of the experiment demonstrated that as the gastric cancer developed, the SDNN and the RMSSD decreased (Hu et al, 2018). This decrease in HRV has been noted with patients that have mid to late stage tumours, this is a result of damage to the autonomic nerves (Walsh and Nelson, 2002).
Clinical Investigations have proven that the HRV can be used as an insight of the extremity of different types of cancers (De Couck and Gidron, 2013). The study analysed, measured different tumour sites and stages. It was discovered that in Gastric Cancer patients, there was a relationship between tumour size and HRV, although when the tumour reached above 2 centimetres, the decrease in HRV was inhibited. The experiment also discovered that the deterioration of the vagal nerves had a direct effect on the spread of the tumour in gastric cancer patients. In conclusion, patients with gastric cancer have a decreased heart rate variability in comparison to the healthy control patients.
Effect of Diabetes Melitus Type One on heart rate variability:
Diabetes Mellitus (DM) type one is a disease that causes the degradation of Beta cells, leading to an insulin deficiency which in turn decreases glucose uptake (Alberti and Zimmet, 1998). Investigations on heart rate variability and type one DM is scarce in comparison to type two DM. Diabetes mellitus (DM) may be accompanied by autonomic nervous system (ANS) dysfunction. Measuring heart rate variability may detect this ANS dysfunction (Malpas and Maling, 1990). Using heart rate variability measurements, it has been noted that patients with diabetes mellitus type one have decreased heart rate variability and also a parasympathetic decrease along with an increase in sympathetic activity in comparison to healthy patients (Guzik et al., 2010; Chessa et al., 2002). Measuring heart rate variability has been shown to prove useful in prognostics and diagnostics by recognising autonomic changes and changes in cardiac rhythm (Khandoker,, 2009; Pivatelli et al., 2012).
Bearing this in mind, the study investigated aimed to demonstrate that heart rate variability indices have a predictive role in type one diabetic patients by identifying autonomic and cardiac rhythm changes (Kastelianne et al, 2017). The study concluded that patients with type one Diabetes Mellitus had irregular HRV, in comparison to the healthy control patients. These changes in HRV are suggestive of an irregular autonomic nervous system which can lead to an increased mortality rate (Schmid et al., 1995).
As an overall review, this study’s findings indicate that the use of HRV indices can be an inexpensive and easy tool to detect diabetes mellitus type one, and to allow clinical assessments of ANS dysfunction, which may be able to decrease mortality rate in patients with type one Diabetes mellitus (Zochodne, 2007).
Effect of age on Heart Rate Variability:
The human body shows us that there are many factors that can affect heart rate variability. The heart rate can change drastically with age. In one such experiment it is seen that ageing is associated with depressed heart rate variability so therefore it can be linked with increased mortality due to lower parasympathetic activity and amplified sympathetic activity.
The study examined healthy individuals with no connection to heart disease or none that were on medication that would affect cardiac function. There was a significant decrease in heart rate variability triangular index with age and no noteworthy alteration change in Root Mean Square of the Successive Differences with age. There was a considerable difference in heart rate variability index in those greater than 70 years of age in contrast to those less than 70 years of age.
There was no significant difference in Root Mean Square of the Successive Differences between the two age groups . The study shows us that ageing reduces the global measure of heart rate variability and suggests reduced responsiveness of autonomic activity to external environmental stimuli with age.(Department of Cardiological Sciences,2004)
Another test carried out experimented on the sympathetic and parasympathetic activity . It was concluded that mutually the low frequency(sympathetic activity) and high frequency (parasympathetic activity) dropped as the age increased. The older age group had lower heart rate variability compared to younger people (Zhang, 2007). When normal children's heart rate variability is contrasted with that of adults , it is evident that children have a considerably higher supine and standing heart rate but a lower supine and standing systolic diastolic blood pressure.
Children also had a drastically higher supine standard deviation of heart rate, supine low frequency ,supine high frequency and standing high frequency powers compared to adults. Children will have a lower ratio of mid frequency to high frequency power while standing. These findings demonstrates a decrease of cholinergic ( nerve cells in which acetylcholine acts as a transmitter) and an increase of adrenergic (nerve cells in which adrenaline or nor adrenaline as a neurotransmitter) modulation of heart rate variability with age (Yerangani et al.,1994).
Effect of gender on Heart Rate Variability:
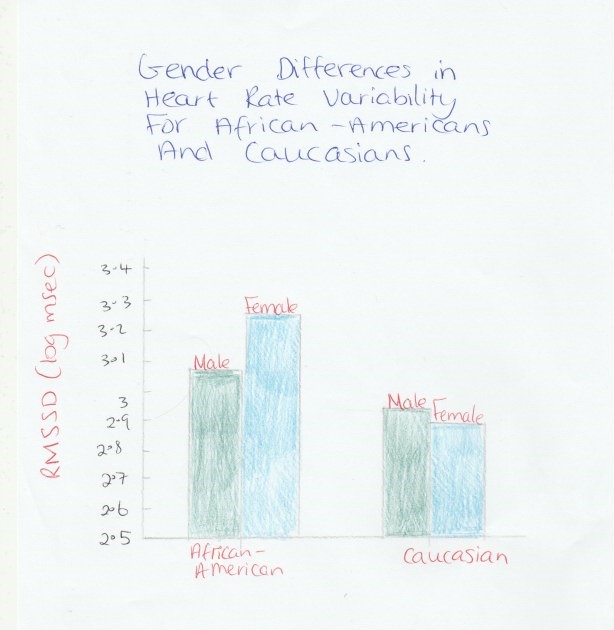
Sex has an important effect on heart rate, but also R-R interval, high frequency, normalised low frequency, normalised high frequency, and low frequency–high frequency ratio. Sex did not seem to affect the standard deviation of the normal- to-normal heartbeats and total power even when there is a significant heart rate changes . It is experimentally demonstrated that age had a greater impact on heart rate variability than sex (see figure 2) (Zhang, 2007).
Effect of ethnic differences on Heart Rate Variabilty:
A study was performed to find any differences in heart rate variability by race and between subjects with high and low blood pressure. The low frequency/high frequency ratio was interestingly higher in caucasians compared to African- Americans. There was a trend for higher low frequency/high frequency ratio in caucasian and African-American with higher levels of blood pressure. It was concluded that healthy white adolescents exhibit increased sympathetic tone compared with that of black adolescents during CV reactivity tests (see figure 2). (Tulane Center for Cardiovascular Health)
Effect of alcohol on Heart Rate Variabilty:
An experiment was carried out to compare alcoholic subjects with non alcoholic subjects. It was proven that alcoholic subjects had faster heart rate and lower pre-imaginary exposure levels of heart rate variability compared to those who were alcohol free. A rise in heart rate variability was observed in the alcoholic group when subjects were exposed to an imaginary alcohol script. Tonic heart rate variability was found to be correlated inversely to negative mood and chronic thought suppression and positively to positive mood. In addition, the uncontrollable sub-scale of the Obsessive Compulsive Drinking Scale was inversely connected to heart rate variability during the imaginary alcohol exposure. (Jon T)
|
Conclusion
In conclusion measuring HRV as a means to detect irregularities of the heart is an easy and inexpensive tool to prevent sudden death in patients with severe diseases. As well as this, the heart rate variability and respiration are interconnected by means of the nervous system, this was proven by two experiments, one which demonstrated the connection under normal conditions, and the other under potentially life threatening circumstances, smoking, which were outlined above. The last point that was discussed was other physiological factors that had an influence on HRV. It was discovered that simple factors such as a persons age, gender, race etc all ultimately had different positive or negative effects on HRV. In summary, analysis of heart rate variability patterns has proven very useful in identifying physiological conditions.
Bibliography:
Alberti, K. and Zimmet, P. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabetic Medicine, 15(7), pp.539-553
Almeida-Santos, M. and Sousa, A. (2015). Heart Rate Variability and Chagas Heart Disease. Arquivos Brasileiros de Cardiologia.
American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. [PubMed]
Anne Kastelianne França da Silva, Diego Giuliano Destro Christofaro, Aline Fernanda Barbosa Bernardo, Franciele Marques Vanderlei, and Luiz Carlos Marques Vanderlei, (2017), Sensitivity, Specificity and Predictive Value of Heart Rate Variability Indices in Type 1 Diabetes Mellitus, available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5389875/ (accessed 3/22/2018).
Berntson, g., thomas bigger, j., eckberg, d., grossman, p., kaufmann, p., malik, m., nagaraja, h., porges, s., saul, j., stone, p. and van der molen, m. (1997). heart rate variability: origins, methods, and interpretive caveats. psychophysiology, 34(6), pp.623-648.
Chessa, M., Butera, G., Lanza, G., Bossone, E., Delogu, A., De Rosa, G., Marietti, G., Rosti, L. and Carminati, M. (2002). Role of Heart Rate Variability in the Early Diagnosis of Diabetic Autonomic Neuropathy in Children. Herz, 27(8), pp.785-790.
De Couck, M. and Gidron, Y. (2013). Norms of vagal nerve activity, indexed by Heart Rate Variability, in cancer patients. Cancer Epidemiology, 37(5), pp.737-741.
Department of Cardiological Sciences, St. George's Hospital Medical School, London, United Kingdom. PubMed.gov. [Online] https://www.ncbi.nlm.nih.gov/pubmed/8945057.
Eckberg, D. L Rea. R. F Andersson, O. K Hedner, T Pernow, J Lundberg. J. M & Wallin. B. G (1988) Baroflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiologica Scandinavica. 133. 221-231.
Furlan R, GuzzettiS, Crivellaro W, DassiS, Tinelli M, Baselli G, CeruttiS, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81: 537-547.
Guzik, P., Piskorski, J., Contreras, P. and Migliaro, E. (2010). Asymmetrical properties of heart rate variability in type 1 diabetes. Clinical Autonomic Research, 20(4), pp.255-257.
Javorka M, Javorková J, Tonhajzerová I, Calkovska A, Javorka K. Heart rate variability in young patients with diabetes mellitus and healthy subjects explored by Poincaré and sequence plots. Clin Physiol Funct Imaging. 2005;25(2):119–127. [PubMed]
John Zhang, MD, PhD, Logan College of Chiropractic, Chesterfield, MO, USA. [Online] http://www.jmptonline.org/article/S0161-4754(07)00122-4/abstract.
Jon T. Ingjaldsson, Department of Clinical Psychology, University of Bergen, Christiesgate 12, 5015 Bergen, Norway. [Online] http://www.biologicalpsychiatryjournal.com/article/S00063223(02)01926-1/abstract.
Kezdi, P,, & Geller, E (1968). Baroreceptor control of postganglionic sympathetic nerve discharge. American Journal of Physiology . 214. 427-435
Khandoker AH, Jelinek HF, Palaniswami M. Identifying diabetic patients with cardiac autonomic neuropathy by heart rate complexity analysis. Biomed Eng Online. 2009;8:3–3. [PMC free article] [PubMed]
Khandoker, A., Jelinek, H. and Palaniswami, M. (2009). Identifying diabetic patients with cardiac autonomic neuropathy by heart rate complexity analysis. BioMedical Engineering OnLine, 8(1), p.3.
Koepchen HP. History of studies and concepts of blood pressure waves. In: Miyakawa K, Koepchen HP, Polosa C, eds. Mechanism ofBlood Pressure Waves. Tokyo/Berlin: Japan Science Society Press/Springer-Verlag; 1984:3-23.
Malliani, A., Pagani, M., Lombardi, F. and Cerutti, S. (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation, 84(2), pp.482-492.
Malpas, S. and Maling, T. (1990). Heart-Rate Variability and Cardiac Autonomic Function in Diabetes. Diabetes, 39(10), pp.1177-1181.
Mayer S. Studien zur Physiologie des Herzens und der Blutgefasse: 5. Abhandlung: Uber spontane Blutdruckschwankungen. Sber Akad Wiss Wien. 1876;74:281-307.
Michael Kessler and Javdat Karimov, (2014), Heart rate variability the why, what and how of HRV and its importance in private practice-Part. Available at: http://ndnr.com/cardiopulmonary-medicine/heart-rate-variability-the-why-what-an d-how-of-hrv-and-its-importance-in-private-practice-part-3-2/ (accessed 3/21/2018).
Mietus, J. (2002). The pNNx files: re-examining a widely used heart rate variability measure. Heart, 88(4), pp.378-380.
Minami J, e. (2018). Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. - PubMed - NCBI. [online] ncbi.nlm.nih.gov. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9931170 [Accessed 21 Mar. 2018].
Montano, N., Ruscone, T., Porta, A., Lombardi, F., Pagani, M. and Malliani, A. (1994). Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation, 90(4), pp.1826-1831.
Park, J., von Karsa, L. and Herrero, R. (2014). Prevention Strategies for Gastric Cancer: A Global Perspective. Clinical Endoscopy, 47(6), p.478.
Peng, R., Yan, W., Zhou, X., Zhang, N., Lin, W. and Zhang, Y. (2015). Time-frequency analysis of heart rate variability during the cold pressor test using a time-varying autoregressive model. Physiological Measurement, 36(3), pp.441-452.
Pivatelli, F., dos Santos, M., Fernandes, G., Gatti, M., de Abreu, L., Valenti, V., Vanderlei, L., Ferreira, C., Adami, F., de Carvalho, T., Monteiro, C. and de Godoy, M. (2012). Sensitivity, specificity and predictive values of linear and nonlinear indices of heart rate variability in stable angina patients. International Archives of Medicine, 5(1), p.31.
RUSHMER, R. (1962). CARDIOVASCULAR DYNAMICS. The American Journal of the Medical Sciences, 243(1), p.130.
Schmid, H., Schaan, B., Cecconello, F., Maestri, T. and Neumann, C. (1995). Proliferative diabetic retinopathy is related to cardiovascular autonomic neuropathy in non-insulin-dependent diabetes mellitus. Diabetes Research and Clinical Practice, 29(3), pp.163-168.
SCHWARTZ, P., PAGANI, M., LOMBARDI, F., MALLIANI, A. and BROWN, A. (1973). A Cardiocardiac Sympathovagal Reflex in the Cat. Circulation Research, 32(2), pp.215-220.
Sociedade Brasileira de Diabetes . Diabetes Mellitus: 2013-2014. São Paulo: AC Farmacêutica; 2014
Songjie Hu, Jie Lou, Youping Zhang and Ping Chen, (2018), Low heart rate variability relates to the progression of gastric cancer. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5842632/ (accessed 3/21/2018)
Tulane Center for Cardiovascular Health, Department of Pediatrics, Tulane University Medical Center, New Orleans, Louisiana, USA. . Pub Med.gov. [Online] https://www.ncbi.nlm.nih.gov/pubmed/9524048.
Walsh, D. and Nelson, K. (2002). Autonomic nervous system dysfunction in advanced cancer. Supportive Care in Cancer, 10(7), pp.523-528.
Witte, H. and Rother, M. (1992). High-frequency and low-frequency heart-rate fluctuation analysis in newborns?A review of possibilities and limitations. Basic Research in Cardiology, [online] 87(2), pp.193-204. Available at: https://www.ncbi.nlm.nih.gov/pubmed/1590741 [Accessed 21 Mar. 2018].
Yeragani, V., Pohl, R., Berger, R., Balon, R. and Srinivasan, K. (1994). Relationship between age and heart rate variability in supine and standing postures: A study of spectral analysis of heart rate. Pediatric Cardiology, 15(1), pp.14-20.
Yi HT, Hsieh YC, Wu TJ, Huang JL, Lin WW, Liang KW, Su CS, Tsai WJ, Wang KY. Heart rate variability parameters and ventricular arrhythmia correlate with pulmonary arterial pressure in adult patients with idiopathic pulmonary arterial hypertension. Heart Lung. 2014;43:534–540. doi: 10.1016/j.hrtlng.2014.05.010. [PubMed] [Cross Ref]
Zochodne, D. (2007). Diabetes mellitus and the peripheral nervous system: Manifestations and mechanisms. Muscle & Nerve, 36(2), pp.144-166.
Figures
Figure 1 Montano, N., Ruscone, T., Porta, A., Lombardi, F., Pagani, M. and Malliani, A. (2018). Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. [online] Moh-it.pure.elsevier.com. Available at: https://moh-it.pure.elsevier.com/en/publications/power-spectrum-analysis-of-heart-rate-variability-to-assess-the-c [Accessed 21 Mar. 2018].
Figure 2: www.researchgate.net (Thomas_Fuller-Rowell, Gayle_D_Love), (2013), Differences in Age-Trends of Autonomic Nervous System Functioning. available at: https://www.researchgate.net/publication/239948556_Race_Differences_in_Age-Trends_of_Autonomic_Nervous_System_Functioning.