1. Abstract
Helicobacter pylori is a spiral-shaped bacterium which is found in the stomach. The most representative microbiological characteristic of Helicobacter pylori is its very strong motility. Another characteristic is that it has a strong urease activity. Helicobacter. Pylori is known as the main factor to cause intestinal type gastric cancer through the sequential process of atrophic gastritis, intestinal meta-plasia and dysplasia. Virulence factor of helicobacter pylori involves CagA, HtrA, BabA, and VacA. Most common symptoms are chronic vomiting, intermittent loss of appetite, and acute gastritis with marked vomiting and loss of appetite with a small probability. There are several diagnostic methods like PCR test, RUT, histological investigation, SAT. There are few treatments like quadruple therapy, sequential therapy, and concomitant therapy.
2. Introduction
Helicobacter pylori is one of the most common bacterial pathogens, but it has not yet been conquered. It is associated with a variety of gastrointestinal disorders, including chronic gastritis, peptic ulcer, gastric marginal B-cell lymphoma, and stomach cancer. Numerous factors such as bacterial toxicity, genotype, host immunity and environmental factors determine disease process and outcome.
3. Helicobacter pylori
3.1 Definition, characteristic of helicobacter pylori
What is Helicobacter pylori?
Although it is known that bacteria cannot live in the stomach due to its strong acidity, it was discovered in 1983 in gastroscopic biopsy tissue that the mucous layer covering the gastric mucosa is inhabited by a bacteria called Helicobacter pylori. Australian doctors Warren and Marshall discovered Helicobacter pylori parasitic in human stomachs and announced that it was an important cause of chronic gastritis, which brought new electricity to identify several digestive diseases, including gastritis, digestive ulcers, stomach cancer and gastric lymphoma.
The fungus was initially called Campylobacter-like organism (CLO), but was named Helicobacter pylori after its helical shape and fatty acid content were found to be different from Campylobacter. "Helico" means a spiral, "bacter" means bacteria, and "pylori" comes from the pylorus of the stomach, the site of the discovery of bacteria. However, it is actually found not only in the stomach but also in the duodenum and mouth. Helicobacter pylori is a spiral-shaped bacterium that is 2~7x0.4~1.2 μm in size (Yim and Kim,2007).
The most representative microbiological characteristic of Helicobacter pylori is its very strong motility. The motility of this fungus can penetrate the gastric mucus layer, which is difficult to penetrate even for compounds, and can live freely within mucus. It uses five to six polar flagella on its body to penetrate mucus and enter into the surface of the gastric mucosa. It stays deep in the mucous layer near the epithelial surface.
Another characteristic is that it has a strong urease activity. Urease is an enzyme that breaks down the components into ammonia and carbon dioxide. Helicobacter pyrolytes effectively break down elements present in the gastric mucosa at low concentrations so that ammonia produced can neutralize the environment around the bacteria, making it safe from strong acids in the stomach.(Gastric acid is acidic than hydrochloric acid, sulfuric acid) It produces acid-inhibitory protein which blocks acid secretion from the parietal cells. It can grow on complex media in microaerophilic, 5% O2, and 10% CO2 conditions, with pH of 6.0-7.0. (Lee and Kim,2014).
Helicobacter pylori is now known as the most infected and most widely distributed bacterium in the world. Not only is it a cause of chronic active gastritis, but it is also found to be linked to various digestive diseases such as peptic ulcers, gastric cancer, and gastric lymphoma. Recently, the World Health Organization (WHO) has defined the fungus as a carcinogen for stomach cancer. Helicobacter pylori is known to be infected in the stomach in about 60% of adults in Korea, and the infection rate varies depending on hygiene facilities and individual hygiene habits(Yim and Kim, 2007). Korea has a higher infection rate than the West, especially in children, which is related to a lot of digestive diseases in Korea. In fact, according to Korean statistics, 94 percent of patients with duodenal ulcers, about 84 percent of patients with stomach ulcers, and about 50 percent of patients with chronic gastritis are infected with the bacterium. In general, the infection rate in our country is 70-80% higher in adults and increases with age (Lee and Choi,2007).
Helicobacter pylori can only live in human stomachs. Until now, possible transmission routes are known to be transmitted through the mouth. Therefore, considering our country's dietary habits, it is assumed that the infection rate in the family will be high. It is known to be carried by human waste in addition to humans. Once infected with this fungus, it will live as a carrier for life or decades.
3.2 physiological background
|
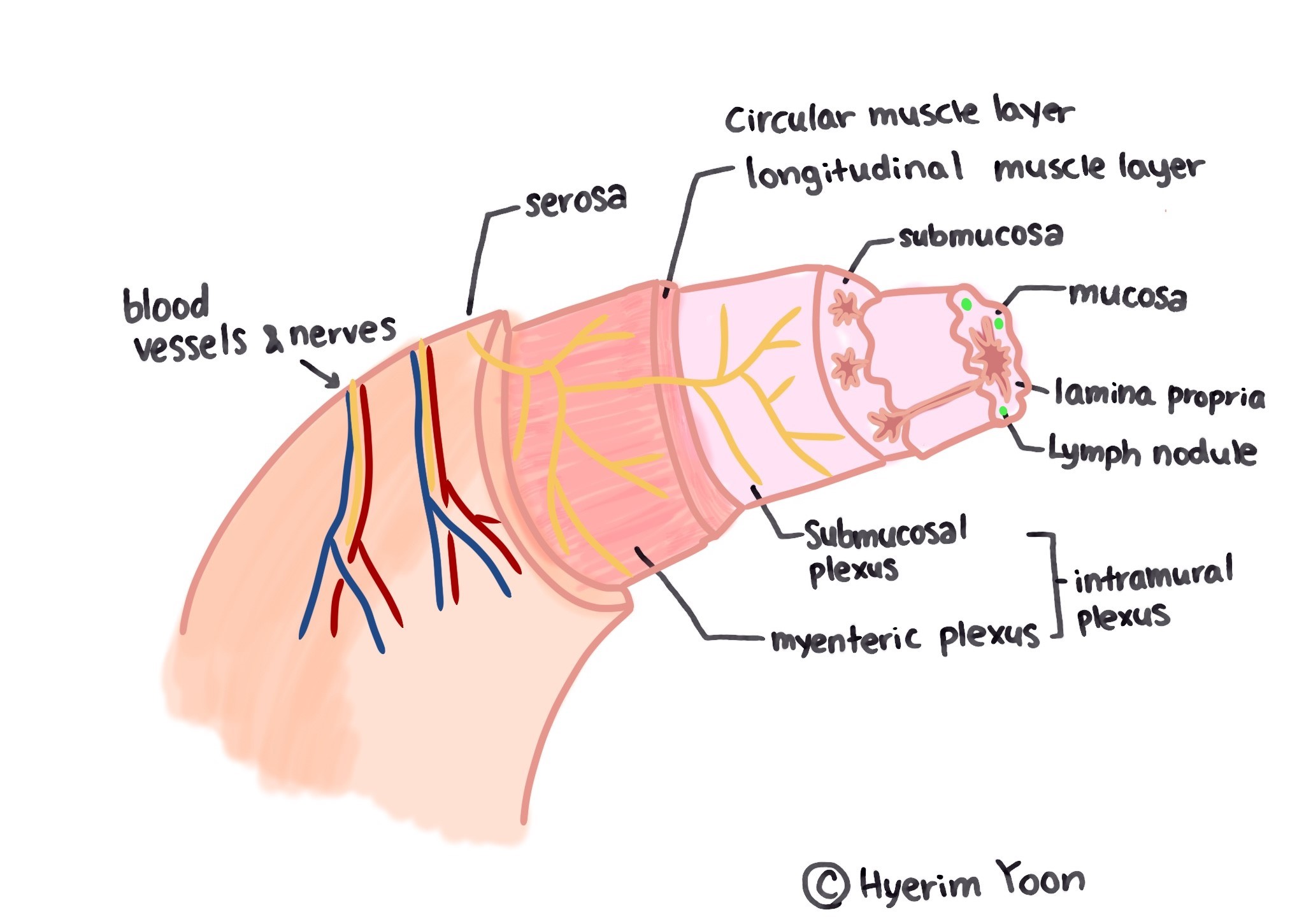
Figure 1 stomach physiology : copyright by Hyerim Yoon |
The stomach is a hollow sac that connects the esophagus and intestines as part of the digestive tract. It is responsible for storing food that has been brought down through the mouth and esophagus for a while and sending it down to the small intestine after some digestion.
The stomach is anatomically divided into a cardia, a fundus, a body, an antrum pylori, and a pylorus. The cardia is directly in contact with the esophageal sphincter and is a passage through which food passes from the esophagus to the stomach. The fundus is a space where food that passes through the esophagus is temporarily stored in the lowest part of the stomach when lying on the left side. The body occupies most of the stomach and is located in the center of the stomach. There are vertical gastric folds on the upper body, which extend the surface area. It becomes flat when there is food and distinct when there is no food.
Between the body of the stomach and the pylorus are called antrum pylori, and stomach ulcers or gastritis are the most common areas. The pylorus is located in the lower part of the upper part and serves to pass the digested food to the duodenum.
The stomach wall consists of four layers: mucosal layer, submucosal layer, muscle layer, and serosal layer. The mucosal layer is divided back into three layers: the mucous epithelium, lamina epithelium, muscularis mucosae.
There is a submucosal layer between the mucosal layer and the muscle layer, where large blood vessels and autonomic nerves are located. Large vessels travel in a direction parallel to the submucosal layer and split into branches in a vertical direction towards the mucosa. The nerves are mainly autonomous, with the parasympathetic nerve distributed in the walls of the digestive tract and the sympathetic nerve distributed outside the digestive tract.
The stomach's muscle layer is thicker than other parts of the digestive tract and is divided into three layers: the inner layer, the mid-circular layer, and the outer longitudinal layer. There is a myenteric plexus(Auerbach plexus), a parasympathetic nerve joint between the circular layer and the longitudinal layer. The serosal layer covers the surface of the stomach with the outermost part of the stomach(Kim,2013). The mucous membrane plate of the gastric mucosa has glands. Mucous neck cells secrete alkaline mucus to protect the gastric mucosa from acid. Main cells secrete pepsin, a protease, and wall cells or acid secretory cells secrete hydrochloric acid and moisture. G cells secrete gastrin and mucus. Wall cells are mainly present in the gastrointestinal tract and are rarely distributed in the pyloric region.
The gastric functions include mechanical digestion, sterilization using acid, and proteolysis through pepsin. The contraction of the muscles that make up the stomach wall causes the food that goes down through the esophagus to mix in the stomach floor. Throughout the stomach, the food changes into a chyme through some digestive processes. Chyme passes through the gastrocnemius sphincter and into the duodenum.
The secretion of gastric fluid and the control of gastric motion involve hormones in the autonomous nervous system and various digestive tracts. The autonomic nerve distributed above acts as a stimulator of gastric motion and secretion, and the sympathetic nerve, on the contrary, exhibits inhibitory action(Kim,2013).
When the food passes through the esophageal sphincter and goes to the stomach, the stomach secretes a proteolytic enzyme called pepsin and a gastric acid that helps the enzyme work. Gastric acid also plays a role in killing or obstructing the action of various bacteria that have entered the mouth. Gastrin increases the secretion of hydrochloric acid and pepsinogen in gastric wall cells and main cells, and promotes gastric movement. It is secreted by G cells in the stomach, which are stimulated by digestive products such as enlarged gastrointestinal cells or incompletely digested proteins. If the pH above falls below 4, the action is suppressed. The cholecystokinin tetrapeptide (CCK) primarily causes the gallbladder to contract, but it also inhibits the peristalsis movement of the stomach and promotes the secretion of pancreatic juice to alkalize or neutralize the lactose((Lee and Kim,2014). Secretin is secreted by the small intestine and is primarily involved in the action of the pancreas, but plays a role in suppressing the movement of the stomach. Gastric inhibitory peptide and enteroglucagon inhibit gastric acid secretion and gastric movement.
3.3 pathophysiology and gastric pathology
Helicobacter.pylori is presumed to be transmitted through the oral or fecal route, and for humans, it has a very high infection rate within the family. In the stomach of individuals infected with H.pylori, H.pylori produces urease, decomposes the urea component of the stomach and reduces the acidity of the surrounding area. H.pylori migrates in the stomach using flagella, and adheres to the gastric epithelial cells through adhesion factors such as BabA.(Lim et al,2013)H.pylori does not always cause acute non-specific digestive symptoms, however, it is reported that H.pylori leads to chronic gastrointestinal diseases around 10-20% of the case.
The complex interaction between virulence factors such as CagA and VacA, which were secreted by Helicobacter. pylori, preservation of the mucous membrane of the body, and other environmental factors are implicated in causing H. pylori-borne diseases. The immune response of the body activates for H. pylori infection, but it is known to accelerate damage to the gastric mucosa rather than removing the source of infection(virulence factors). Helicobacter. pylori causes antrum-predominant gastritis, promotes gastrin secretion, inhibits somatostatin secretion, and leads duodenal ulcer. It is also reported that H. pylori inhibits gastric acid secretion, causes pangastritis or corpus predominant gastritis, and leads gastric ulcer and gastric cancer.
Helicobacter. Pylori is known as the main factor to cause intestinal type gastric cancer through the sequential process of atrophic gastritis, intestinal meta-plasia and dysplasia. It is reported that H. pylori was the main factor of gastric cancers in 71-95% cases, but conversely, not all H. pylori-infected patients got gastric cancer (less than 1% of H. pylori-infected patients had gastric cancer). It is estimated that cancer occurs due to multifactorial etiology such as genetic factors, diet, and the characteristics of the bacteria themselves.
Primary gastric displacement zone B-cell lymphoma accounts for 1-7% of malignant tumors in the stomach, but is known to be the most common type, accounting for 50-60% of all gastrointestinal lymphomas. Up to 98% of gastric B-cell lymphomas and 30% of H. pylori infections produce lymphoid follicles through the inflammatory reaction of the mucous membrane, which is presumed to be a precursor to marginal B-cell lymphoma.
In about 60% of patients complaining of symptoms of functional dyspepsia are diagnosed as functional dyspepsia, it shows a prevalence rate of 11-14% and no organic diseases are found through endoscopy and/or radiological examination, etc. However, the diseases such as Postprandial discomfort, bloating, epigastric pain, heartburn, etc. occur chronically and recurrently.
Research says that 40 to 70% of functional indigestion is caused by H. pylori infection, and chronic gastric inflammation by H. pylori infection was suggested as a mechanism of the functional dyspepsia occurrence. Inflammatory reaction activated by chronic infection causes abnormalities in gastrointestinal motility and awareness due to hyper hormones and changes in gastric acid secretion.
|
Figure 2 helicobacter : copyright by Bokyung Seo |
3.4 clinical presentation
3.4.1 symptoms
As aforesaid, Helicobacter bacteria is presumed to be transmitted through oral-oral and fecal-oral routes. However, it is speculated and not clearly confirmed, and vector transmission can occur. In a specific area, waterborne sources of infection may be important to Helicobacter bacteria infection. Helicobacters have the potential to infect animals as well as humans.
Helicobacter species have been identified in dog saliva and tartar, and possibly have oral-to-oral transmission. It supports the concerns about oral-to-oral transmission between dog-to-dog or dog-to-human. H. pylori is rarely isolated in cats (rather than dogs). Although, the possibility of animal infection is a reasonable concern. In the human body, H. pylori infection has been associated with an increased risk of peptic ulcer disease and gastric cancer. H. canis, H. felis, H. heilmannii infections are believed to be acquired in dogs and cats, and have been reported along with associated bacteremia, peptic ulcer disease, and gastritis. Anthropozoonosis (human-animal transmission) can occur in cats.(McColl,2010)
Several types of Helicobacter have been found in dogs and cats. In dogs, H. felis, H. bizzozeronii, H. salomonis, H. bilis, H. heilmannii, and Flexispira rappini were observed. For cats, H. felis, H. pametensis, H. pylori, H. heilmannii. About 41%-100% of healthy cats and 57%-100% of vomiting cats have Helicobacter-like organisms.(Recordati et al,2009) Gastritis caused by Helicobacter bacteria varies in severity and is often lymphoplasmacytic. Whether gastric erosion and ulcers occur in infected dogs or cats is still under discussion.It is still unknown whether human pathophysiological mechanisms, namely the production of ammonia by bacterial urease and other secretions that damage epithelial cells and induce gastric acid secretion occur in dogs and cats.
Helicobacter species are not critically pathogenic in all dogs and cats. Helicobacter is often found accidentally in histological examination of a gastric biopsy. The diagnosis of Helicobacter infection is difficult to confirm by gastric biopsy, and the pathogenic importance of dogs and cats is controversial. If possible, an underlying diagnosis should be sought in animals with gastrointestinal symptoms or ulcers.(Eckel,2010)
Helicobacter organisms can be identified even in healthy animals. Most common symptoms may include chronic vomiting, intermittent loss of appetite, and acute gastritis with marked vomiting and loss of appetite (with gastric palsy) with a small probability. These cases are reported to be more common in hospitalized dogs and in dogs who have recently been exposed to the stool of other dogs in kennels, salons or veterinary clinics.(Bruce,2010)
3.4.2 diagnostics
Various methods are used to carry out diagnostics of helicobacter pylori. These methods can be divided into 2 types. The first type is the ones that can take place invasively using endoscopy and another type can be characterized as a noninvasive technique which is usually seen as a more preferred method. One of the most common invasive methods is PCR (polymerase chain reaction), RUT (rapid urease test), and simple histological investigations(Breno et al, 2019). These tests can be implemented using endoscopy on tissue such as gastric tissue. There are also some alternative noninvasive approaches instead of invasive ones, including UBT(urea breath test) and SAT(Stool antigen test).
Invasive methods
PCR (polymerase chain reaction)
PCR is a mechanism to make numerous copies of certain DNA segments allowing one to augment the DNA samples to a large, quantifiable amount. PCR can be used to detect H.pylori DNA in samples in both invasive and non-invasive ways. Gastric, saliva, and dental plaques are one of the most common samples for invasive PCR. However, invasive types of PCR have been proven to be more effective. Before any steps of PCR can be initiated DNA has to be purified, isolated, or extracted. There is high diversity in DNA extraction mechanisms so one has to unify the method in one type in order to obtain reliable results. The first step of the PCR is denaturation where the DNA template helix gets unwinded via disruption of complementary hydrogen bonds(Jose, 2018). The second step is the annealing step to anneal the primers to the ss DNA template by lowering the temperature subsequently producing stable hydrogen bonds after the primer matches with the template. The final step is the extension step is facilitated by enzymes called DNA polymerase and Taq polymerase at a certain optimum temperature. In PCR testing, the positive control is crucial in order to make sure that the primer of the PCR process is adequate for normal propagation and to make sure that the PCR assay itself is precise so that one doesn’t have to repeat the test. Also, the type of primer used in the PCR is of highlighted importance since the sensitivity to H.pylori DNA depends on the type of primer used. Recently, a specific type of rRNA and 23s rRNA have been proven to be the most effective primer (Breno et al, 2019). PCR is exceptionally beneficial in detecting certain bacterium in environmental samples with high specificity and accuracy for a short period of time. Also, it can observe any mutations to inform one about antibiotic resistance. Possible drawbacks can be lack of protection against contamination, costly, and lack of information about antibiotic susceptibility.
RUT (rapid urease test)
RUT is also known as CLO (Campylobacter-like organism test) which utilizes Helicobacter’s ability to secrete urease enzyme to catalyze the reaction of converting urea to ammonia and CO2. The main goal of this test is to identify urease in a given sample. Large quantities of urea can be detected in RUT from activities of urease that can be multiplied on samples from invasive methods. Due to the presence of ammonia, there’s an increasing pattern in the PH which can be detected with the PH indicator with notable color change. Low sensitivity of RUT can be detected in patients with achlorhydria or from a high intake of antibiotics and PPI. (Breno et al, 2019) Moreover, there are special urease producers, such as Streptococci and Staphylococci, in the stomach. RUT presents high specificity and sensitivity with relatively low cost and less time-consuming.
Histological investigations.
Histological examinations are the standard way for Helico pylori diagnostics as they are simple and used universally. Accurate histological investigations can provide information about possible H pylori colonization with bacterial concentration density and potential malignancy of the infection(Jose, 2018). Specific dyes such as Toluidine blue, Hematoxylin-eosin, and Giemsa can be used to identify bacterial infections. Even though this method is widely used, fluorescent microscopes are required and a high percentage of false-negative results can be detected. Noninvasive method
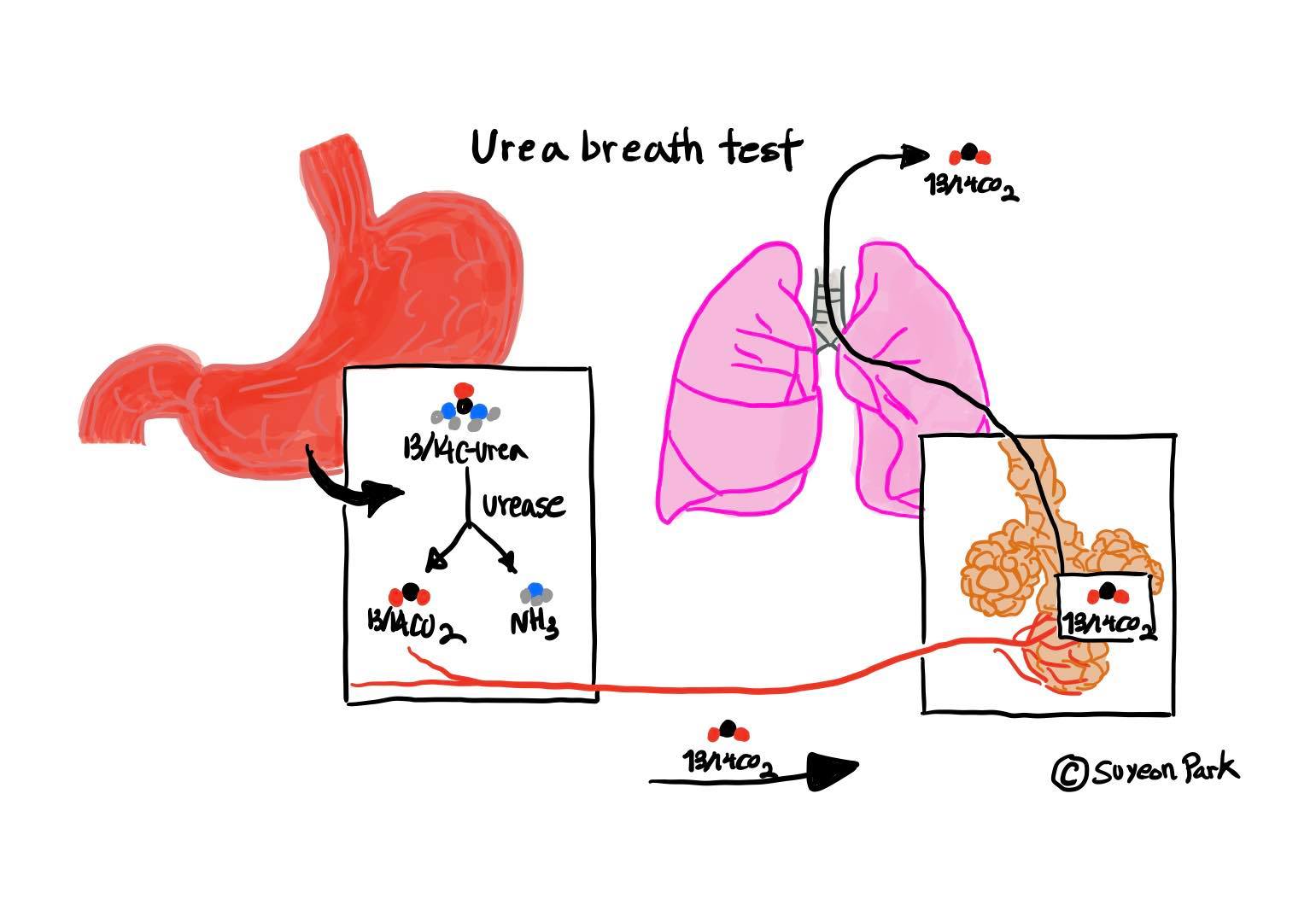
UBT (urea breath test)
|
Figure 3 Urea Breath Test : copyright by Suyeon Park |
UBT is a rapid diagnostic procedure that shares some traits with the RUT test. They both use the ability of the H. pylori in dispensing urease for the breakdown reaction of carbon dioxide and urea. UBT can be done when an individual is given a pill containing 14C which is a slightly radioactive isotope (Lee and Kim 2014). UBT test turns out to be positive when urea is detected in the breath since breath contains isotope containing carbon dioxide. UBT is used universally and is safe to use for children with high sensitivity, however, it doesn’t provide any information about antibiotic resistance(R Sapierzyninski, 2006).
SAT (stool antigen test)
SAT seeks for any possibility of H pylori antigen occurrences in one’s stool. It is usually used as an alternative non-invasive method instead of UBT. Even though it’s considered an alternative choice, SAT has some advantages compared to UBT (W Prachasilpchai et al, 2007). First SAT can be stored at home by the patients and preparatory materials are easily accessible and not costly with the low necessity for high tech equipment. According to recent research implementing SAT with antibodies has been determined to be the best method to plan out the eradication of the bacteria.
3.4.3 treatments
The treatment of Helicobacter pylori can be divided depending on the various body parts with specific resistance degrees to clarithromycin which is one of the main antimicrobial treatments against H pylori. There are certain body parts with low or high resistance to clarithromycin so slightly different treatment plans have to be implemented to adjust to such differences(Jose, 2018). Generally, there is no single type of antibiotics that can completely cure H pylori infection, instead, several different antibiotics are taken together to increase the efficiency of the effect. The effect depends on the certain antimicrobial strain inside the bacteria and also depends on how tolerable the patients’ bodies are to it. Along with the effect of antibiotics, a drug called bismuth subsalicylate is used to deteriorate the bacteria (Jose, 2018). Other than factors that influence the bacteria, other medicines are used to prevent the stomach from producing more acid for a measure to prevent the gastric acid from propagating. Another aspect to be aware of is histamine being the positive regulator of gastric acid release. Histamine is released by gastrin so drugs, such as ranitidine, have to be administered to alleviate the effect of the infection by blocking gastrin activity(Sargyn, 2013). These medicines include pantoprazole, omeprazole, and esmeprazole. The main examples of standard treatment to H pylori infection are as follow :
Quadruple therapy
Quadruple therapy includes a combination of antibiotics, PPI, bismuth subsalicylate, clarithromycin, and tetracycline who are injected twice per day for one week (Jose, 2018). These antibiotics are used to directly kill the bacteria inside but at least 2 of the medicines have to be incorporated to give full impact against the presented infection. This therapy especially is proven to be highly effective against regions where there is relatively high level of antibiotics resistance (Lee and Lee 2018).
Sequential therapy
Sequential therapy also uses a combination of drugs except it doesn’t involve bismuth subsalicylate but clarithromycin is also corporated with PPI and amoxicillin that are given fives days after the triple therapy. For sequential therapy, it is characterized as a dual therapy of the Helicobacter (Lee and Lee 2018). It's not considered to be the perfect regimen for eradication but the effect of sequential therapy has been proven more effective than other methods, especially in children.
Concomitant therapy
This therapy is used as an alternative to sequential therapy characterized as administering different antibiotics simultaneously containing bismuth subcitrate potassium, tetracycline, and metronidazole. It can also be described as quadruple therapy due to the number of drugs used and its eradication effect can reach up 90% presenting high efficacy against ongoing infection(Sargyn, 2013).
3.4.4 virulence factors
Bacteria’s virulence factor points to the fact that it allows virulence to augment a colony in a host organism and to replicate itself to subvert the host’s immune system. Finding the origin and types of virulence factors allows one to learn more about the bacteria’s ability and efficacy in order to create corresponding antibiotics against such infection. There is more than one virulence factor in H pylori and each of them has distinguishable traits that support the bacteria in its function and debilitate the host’s defense against it (Jafarzadeh, 2010). One of the main examples of virulence factors are as follow:
CagA (Cytotoxin associated gene a)
CagA is an oncoprotein (bacterium-derived oncoprotein) that gets translocated on the host to facilitate the process of gastric inflammation and is especially associated with the lymphoid tissue in the gastric mucosa. After CagA gets translocated, it increases the possibility of gastric cancer and lesion which can lead to cancer later(Jafarzadeh, 2010). CagA ultimately alters the signal transduction pathway that expedites the conversion of gastric epithelial cells that cause gastric ulcers. The level of severity is proportional to the level of translocation degree in a host cell.
Htr A (high-temperature requirement a)
Htr A is a type of protease secreted from H pylori that induce the cleavage of extracellular material and junctional proteins in order to expose the receptors located at the basolateral side of the host cells (Breno et al, 2019). Suppressing the gene coding Htr A is efficient in the eradication process since Htr A helps the bacteria to survive in stressful environments in terms of PH and temperature of the toxic gastric mucosa environment of our body.
BabA (blood group antigen adhesin a)
BabA is an adhesin (adherence factor against the given host cell, cell attachment action) that is present on the membrane of the H pylori to get adhered to the specific receptor of the host cell which can be glycoproteins or glycolipids causing inflammation in the stomach. The action of the BabA from the corresponding gene regulates and supports in the formation of the H pylori colony in the stomach (Shanta met al, 2014).
Vac A (vacuolating cytotoxin a)
This virulence factor is specialized in deteriorating the gastric epithelial cell further inducing inflammation. The process can be initiated by inducing vacuole formation in epithelial cells. At the extracellular surface of the host, this virulence factor tends to attach to the sphingomyelin lipids partially. VacA gets distributed inside the cell contained in the membranes of vacuoles(Breno et al, 2019). Also, it induces a form of assembly creating an anion channel of chloride ions in the inner membrane of the mitochondria of the target cell. These anion channels have distinct oligomeric structures causing the formation of massive vacuoles inside the host cell(Jafarzadeh, 2010).
4. Conclusion
Helicobacter pylori initially called Campylobacter-like organism, is a bacteria with a noticeable helical shape that causes infection in the gastric environment by eliciting different translocated virulence factors such CagA and VacA to get in contact with gastric mucosa, subverting the immune system of the host. Being one of the most widespread bacteria in the world, H pylori's agile motility and its urease activities allow it to effectively infect the host and ultimately leads to chronic antrum predominant gastritis, peptic ulcers, gastric lymphoma, and even gastric cancer. Different types of invasive and non-invasive diagnostics measures can be implemented so careful consideration is required for both the patients and physicians to choose the best diagnostic mechanism for an individual's current health and status. Treatment can be followed up with different therapies that involve more than one drug and antibiotics, so it might take more than one trial of therapy to fully eradicate the bacteria from the patient and to alleviate the current effect of bacteria on the gastric mucosa. Even though complete eradication of H pylori is seen as a predicament due to H pylori's flexibility to antibiotics effects, one can be fully treated by going through potential treatment plans that are made to have high efficacy against emerging virulence factors. Not only new diagnostics and therapies were developed in the process, due to its persistent nature, but H pylori have also propagated the scientists to find and expand a new field of discovery about the gastric environment and the associated diseases leading to new investigations of different physiological mechanisms of the stomach. Due to such intensive research about the bacteria and corresponding knowledge about the visceral environment, we now can understand better and have a brand new impression about the previously seen as vague gastric habitat and metabolism ultimately preventing the potential future development of H pylori infection on human population.
5. References
Kim CG(2013): Helicobacter pylori and Hematologic Diseases. The Korean Journal of Medicine. 84.6: 669-673.
McColl KE(2010): Clinical practice. Helicobacter pylori infection. N Engl J Med. 362:1597-1604.
Yim JY, Kim N, Choi SH, et al(2007): Seroprevalence of Helicobacter pylori in South Korea. Helicobacter.12:333-340.
Lim SH, Kwon JW, Kim N, et al(2013): Prevalence and risk factors of Helicobacter pylori infection in Korea. nationwide multi- center. BMC Gastroenterol.13:104.
Lee JY, Kim N(2014): Future trends of Helicobacter pylori eradication therapy in Korea. Korean Journal Gastroenterol. 63:158-170.
Lee JH, Lee YC, Jeon SW, Kim JW, Lee SW(2009): Korean College of Helicobacter and Upper Gastrointestinal Research. Korean Association of Gastroenterology. Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 54:309-317.
W Prachasilpchai, et al(2007): Diagnosis of Helicobacter spp. infection in canine stomach. J Vet Sci. 8:139
C Recordati, et al(2009): Spatial distribution of Helicobacter spp. in the gastrointestinal tract of dogs. Helicobacter . 14:180
R Sapierzyniski, et al.(2006): The diagnosis of gastritis and Helicobacter like organisms infection in endoscopic biopsies of the canine gastric mucosa. Pol J Vet Sci. 9:17
Shantha A, Taslima T L, Jazmin G, Irina V P, Ellen J B, Victor E B (2014): Effect of Helicobacter pylori on gastric epithelial cells. J Pharm Bioallied Sci. 20(36): 12767–12780
Breno B, Filipe S, Aline S, Vinicious A, Maria S, Pedro M, Mariana S, Fabricio M (2019): Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 25(37): 5578–5589
Eckel RH(2010): The metabolic syndrome. Lancet. 375 : 181-183.
Bruce KD(2010):The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 140 : 648-652.
Jafarzadeh A(2010): Serum concentrations of Helicobacter pylori IgG and the virulence factor CagA in patients with ischaemic heart disease. East Mediterr Health J. 16 : 1039-1044.
Sargýn M(2003): Type 2 diabetes mellitus affects eradication rate of Helicobacter pylori. World J Gastroenterol. 9 : 1126-1128
Jose Y (2018) : Diagnosis of Helicobacter Pylori Using Invasive and Noninvasive Approaches. 10: 2090-3057