Adrenocorticotrop hormone normal function and Addison´s Disease
Contents
Adrenal gland
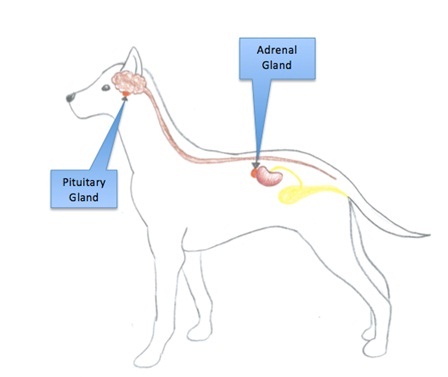
ACTH is a adrenocorticotropic hormone produced in adrenal gland. The adrenal gland is paired and located near the kidneys. The gland consists of an outer cortex and an inner medulla, which produces steroid hormones and catecholamines, respectively. The cortex originates from mesoderm while the medulla has ectodermal origin and is a part of the sympathetic nervous system (Bowen,2002). The cortex consists of 3 zones or layers (see figure 1), (Burkit et al,1993).
1. Zona glomerulosa – outermost layer - Main site for mineralocorticoid production, mostly aldosterone
2. Zona fasciculata - Main site for glucocorticoid production, mostly cortisol and corticosterone.
3. Zona reticularis – inner most layer - Main site for aldosterone and estrogen production
Figure 1. Anatomical location of regulatory and secretory glands involved in ACTH production.
Normal function of ACTH
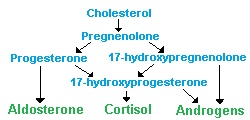
The ACTH has several functions regarding synthesis and secretion of steroid hormones, such as glucocorticoids, mineralocorticoids as well as androgens and estrogens. When ACTH is present and active, it will effect adrenal cortex stimulation and secretion by increasing the conversion of cholesterol to pregnenolone (see figure 2), resulting in increased mineralocorticoid synthesis. By increasing the cAMP level, synthesis of glucocorticoids synthesis in Z. Fasiculata and Z. reticularis increases.
Increased ACTH level also has an increasing effect on melanocyte stimulating activity, due to melanocyte stimulating hormone (MSH) being in the amino acid sequence of ACTH. Both ACTH and Melanocytes have the same precursor (Pre - POMC), (Sjaastad et al, 2010), (Bowen, 2001).
Figure 2: Steroid hormone production pathways of the adrenal gland
Synthesis
The adenocortiotrop hormone (ACTH) is synthesized from pre-pro-opiomelanocortin (Pre-POMC). The Pre-POMC is a hormone precursor, found in the pituitary gland. Removal of a signal peptide from (PRE-POMC), produces the POMC, which then is cleaved by prohormone convertases,undergoes different modifications and yields various polypeptide fragments (see figure 3) such as (Sjaastad et al, 2010), (National Library of Medicine,1993).
- ACTH
- Alfa , beta, gamma Melanocyte Stimulating Hormone
- Gamma lipotropic hormone
- Corticotropin-like Intermediate Peptide (CLIP)
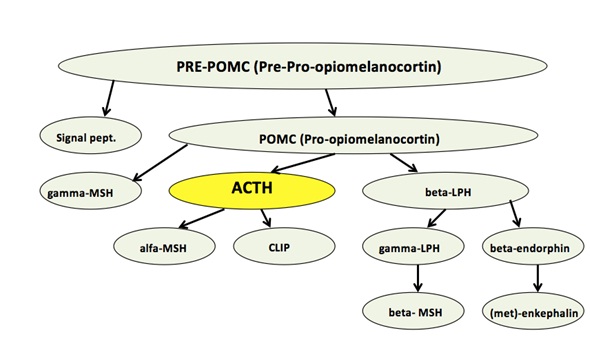
Figure 3. ACTH synthesis pathway
Regulation
ACTH is regulated by corticotrop releasing factor (CRF) which is secreted and released from the integrator of the endocrine system, the hypothalamus, according to classical-feedback system.
CRF also stimulates the cleaving of all the processes mentioned above and is a stimulator of POMC synthesis. Exogenous, endogenous effects, acetylcholine and serotonin may induce the secretion of CRF from the hypothalamus. Corticotrop releasing factor reaches the anterior pituitary gland and ACTH will be released. ACTH will then stimulate the synthesis of steroid hormones in adrenal cortex.
The increase in concentration of glucocorticoids secreted from the cortex, will inhibit the CRF secretion from the hypothalamus. Which in turn leads to decrease of ACTH secretion from anterior pituitary gland. This is through both the long and short feedback pathways (see figure 4), (Sjaastad et al, 2010), (Swenson,1984).
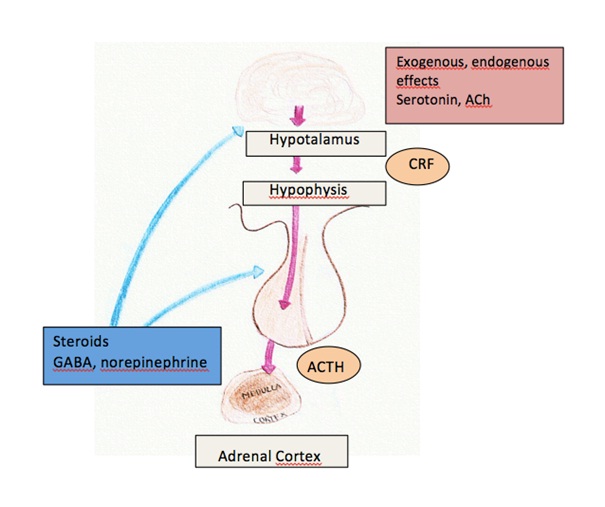
Figure 4: Classical feedback pathway of ACTH
The idea behind the classic feedback mechanism is that elevated levels of hormones produced by the body inhibits their own central production. There is three pathways that the feedback mechanism is grouped (see figure 5).
1. Long Feedback - communication between a peripheral gland and the hypothalamus
2. Short Feedback - peripheral gland and the pituitary gland
3. Ultra Short Feedback - hypothalamus and the pituitary gland
All of these pathways are essential in maintaining inner hormone balance within the body. Changes in environment, physiological state or stress may elicit a change in hormone production.
Addison’s Disease
This is an endocrine disorder first named and described by Dr. Thomas Addison and exerts its most prevalent effects in the adrenal gland and thus disrupts its functions. The result of this disorder is the failure in the ability to produce enough steroid hormones which are produced in the adrenal cortex. Glucocorticoids and mineralocorticoids are the two main classes of steroid hormones whose production is effected with the disease. They are essential in the maintenance of metabolic and electrolyte balance within the body, hence being crucial in homeostasis.
Addison’s disease is seen as a “Primary Adrenal Insufficiency". This means a direct problem with the adrenal gland itself. The steroid hormone synthesis of the adrenal cortex is under regulation of Adrenocorticotrop Hormone (ACTH) which is produced in the pituitary gland. The release of this hormone is under the control of Corticotrop Releasing Hormone (CRH) which is released from the hypothalamus. The entire process of regulation here is controlled by the classic feedback method
Primary Adrenal Insufficiency is clearly distinguished as Addison’s disease as it specifically refers to an insufficiency in the adrenal gland’s functions itself and its inability to carry out steroid hormone synthesis. Secondary and tertiary insufficiencies are defects in ACTH and CRH production and deliverance respectively and are different as they refer to inefficiencies in production of precursors to adrenal cortex hormones in the hypothalamus and pituitary glands, not the adrenal cortex (Klein et al, 2010).
The accumulation of all these factors can result in what is known as an Addison’s Crisis (Klein et al, 2010), (Rennert, 2011):
- Severe drop in blood pressure
- Hypoglycemia – plasma glucose levels drop
- Hyponatremia – plasma sodium levels drop
- Hyperkalemia – plasma potassium levels increase
- Hypercalcemia – plasma calcium levels increase
- Coma
Mineralocorticoids are the essential in electrolyte and water regulation of the distal convoluted tubules of the kidney. They stimulate the reabsorption of Na and secretion of K, hence facilitating homeostasis. The main hormone is Aldosterone which acts here. Addison’s disease leads to the disrupted production of this hormone and as a result disrupted regulation of metabolic functions in the kidney which is of major clinical relevance in the animal.
Causes
Causes of adrenal insufficiency are grouped according to the way that they influence the adrenal gland to fail in its regular function:
1. Adrenal Dysgenisis This is where the adrenal gland itself is not entirely developed during growth from an embryo and is mostly referred to as being genetic. When an infant is born the adrenal gland is not entirely developed resulting in the inability to produce the required hormones.
2. Impaired Steroidogenisis To form the steroid hormones within the adrenal gland cholesterol is required as it is a precursor to both mineralocorticoids and glucocorticoids but there is several ways in which it cannot function:
- Inability to synthesise cholesterol due to low occurrence of enzyme (7-DHC reductase) which cleaves precursors to produce cholesterol. Its is a endogenous enzyme required for the further production of cholesterol.
- Disorder in absorption of fat and fat soluble vitamins from foodstuffs - Malabsorption. This can be due to a number of factors like low levels of bile, digestive enzymes and HCl and also the over abundance of microbial bacteria in the GI tract. All of these lead to a deficiency in cholesterol and hence adrenal steroid synthesis.
- Lipid Congenital Adrenal Hyperplasia: It arises from defects in the earliest stages of adrenal cortisol synthesis: the transport of cholesterol into the mitochondria of the cells of the adrenal cortex and the conversion of cholesterol to pregnenolone—the first step in the synthesis of all steroid hormones.
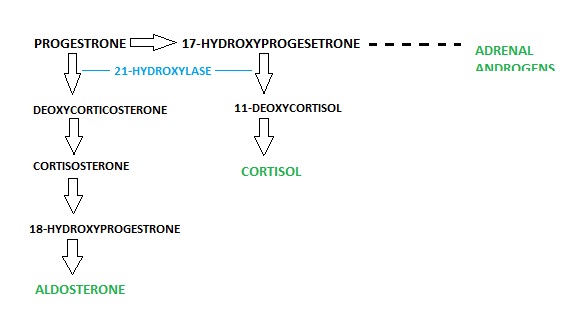
3. Congenital Adrenal Hyperplasia Autosomal recessive disease resulting in mutations in genes for transcription of enzymes required to mediate biochemical steps of cortisol production. Without cortisol, there is a failure of negative feedback and excessive secretion of corticotropin-releasing hormone from the hypothalamus and ACTH from the anterior pituitary. Continued secretion of ACTH causes unremitting stimulation of the adrenal cortex, leading to hyperplasia (an increase in the number of cells in that tissue). Immune reaction on enzyme 21 – Hyroxylase where own body turns on its own enzyme synthesis hence stopping further steroid hormone synthesis is the most common (around 90% of cases), (Bowen, 2001), (Dunlop et al, 2004).
Figure 5: Shows the normal pathway of adrenal steroid synthesis
4. Corticosteroid Withdrawal: This is a result of elevated usage of high dose exogenous steroids. The use of these leads to surpression of hypothalamic CRH and pituitary ACTH production via classic feedback. After prolonged use they can lead to adrenal atrophy (shrinking).
Symptoms
Fatigue, muscular weakness, fever, vomiting, low blood pressure, drastic changes in plasma metabolite levels. Hyperpigmentation is also a symptom as ACTH having the same precursor as Melanocyte Stimulating Hormone (MSH). When the body recognises and a drop in gluco & mineralocorticoid levels it increases its ACTH production, indirectly leading to more MSH being produced. It is also specific to Primary Adrenal Insufficiency as Secondary and Tertiary insufficiencies do not show these symptoms (Klein et al, 2010).
Treatment
Addison’s disease is seen much more in dogs than it is in humans (x100 times more common) and it is also noted that most cases of the disease are seen in females (about 70% of cases). Hypoadrenocorticism is treated with exogenous hormones. These hormones artificially maintain adrenal steroid hormone levels within the animal. During steroid treatment, the adrenal glands do not function fully. The body senses the levels of the exogenous steroids in the system and therefore does not signal for additional production. If an animal begins to display signs of improving the usual protocol for stopping steroid medications is not to eliminate them suddenly, but to withdraw from them gradually in a "tapering off" process, which allows the production to adjust to normal. But if steroids are abruptly withdrawn, the dormant adrenal glands may not able to reactivate, and the body will need to have its adrenal steroid hormones replaced by medication. Most of the medications used in the therapy of hypoadrenocorticism cause excessive thirst and urination. It is absolutely vital to provide fresh drinking water for a canine suffering from this disorder in their recovery (Klein et al, 2010), (Bruyette, 2008).
List of references
Litterature
- Burkitt H.G., Young, B., Heath, J. W. (1993): Weather´s Functional Histology A text And Colour Atlas. Churchill Livingstone
- Dunlop H.R., Malbert, C-H., (2004): Veterinary Pathophysiology. Blackwell publishing
- Sjaastad V.O., Sand, O. and Hove, K. (2010): Physiology of Domestic Animals. Scandinavian Veterinary Press
Swenson J. M. (1984): Dukes Physiology Of Domestic Animals 10th edition. Cornell University Press
Articles
Bowen R. (2001): Stereoidogenesis [internet]. Colorado State University. 2001 Oct 20 [cited 2012 Nov 2]. Available from:http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/basics/steroidogenesis.html
Bowen R. (2002): Functional Anatomy of the Adrenal Gland. Thyroid & Adrenal Research Institute, Scottsdale, AZ. 2002 Jul 5 [cited 2012 Nov 2]. Available from: http://www.thyroidinstitute.org/adrenal_gland_anatomy_physiology.html
Bruyette D.S. (2008): Managing atypical and critical cases of primary hypoadrenocorticism in dogs. DVM360, CVC, Veterinary Medicine, 2008 Dec [cited 2012 Nov 2]. Available from: http://license.icopyright.net/user/viewFreeUse.act?fuid=MjU5MDkyMA==
Klein S.C. and M.E. Peterson (2010): Canine hypoadrenocorticism: Part I. The Canadian Veterinary Journal. Canadian Veterinary Medicine Association (CVMA), 2010 Jan [cited 2012 Nov 7]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2797351/
Klein S.C. and M.E. Peterson (2010): Canine hypoadrenocorticism: Part II. The Canadian Veterinary Journal. Canadian Veterinary Medicine Association (CVMA), 2010 Feb [cited 2012 Nov 7]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2808283/
McGonigle K.M., J.F. Randolph, S.A. Center, R.E. Goldstein (2012): Mineralocorticoid Before Glucocorticoid Deficiency in a Dog with Primary Hypoadrenocorticism and Hypothyroidism. Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY. 2012 Nov 12 [cited 2012 Nov 16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23033468
National Library of Medicine (NLM), Bethesda, MD., (2011): Library of Medicine - Medical Subject Headings MeSH, Pro-Opiomelanocortin [internet]. 1993 Jan 10 [updated 2011 Aug 31]; [cited 2012 Nov 3]. Available from: http://www.nlm.nih.gov/cgi/mesh/2011/MB_cgi?mode=&term=Pro-Opiomelanocortin.
Rennert N.J.(2011): Addison's disease. MedlinePlus. National Library of Medicine (NLM), Bethesda, MD. 2011 [updated 2011 Nov 12]; [cited 2012 Nov 3]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000378.htm
Cian Kennedy, Elisabeth Faureng, Vera Faaberg
All Pictures copyright (C) Cian Kennedy, Elisabeth Faureng, Vera Faaberg