Itt írjon a(z) Immun_RNA_therapy-ról/ről
Immunological background of RNA therapy
Introduction
This essay will discuss the immunological background of RNA therapy. It will give a brief introduction to what RNA therapy is, how it works and how it is clinically beneficial. The immune system consists of a complex unity of cell types whose main goal is to protect the organism and maintain homeostasis. Single-cell RNA sequencing technologies have changed the ability to study immunology. RNA therapy may be applied to several components of both the innate and the adaptive immune systems. It can be divided into several subtypes with a great variety in function. RNA interference (RNAi) is of great significance for treatment of various pathological conditions. Messenger RNA (mRNA) is used in the making of vaccines because it can trigger the protein synthesis within the cells (Chen et al., 2019) .
What is RNA therapy?
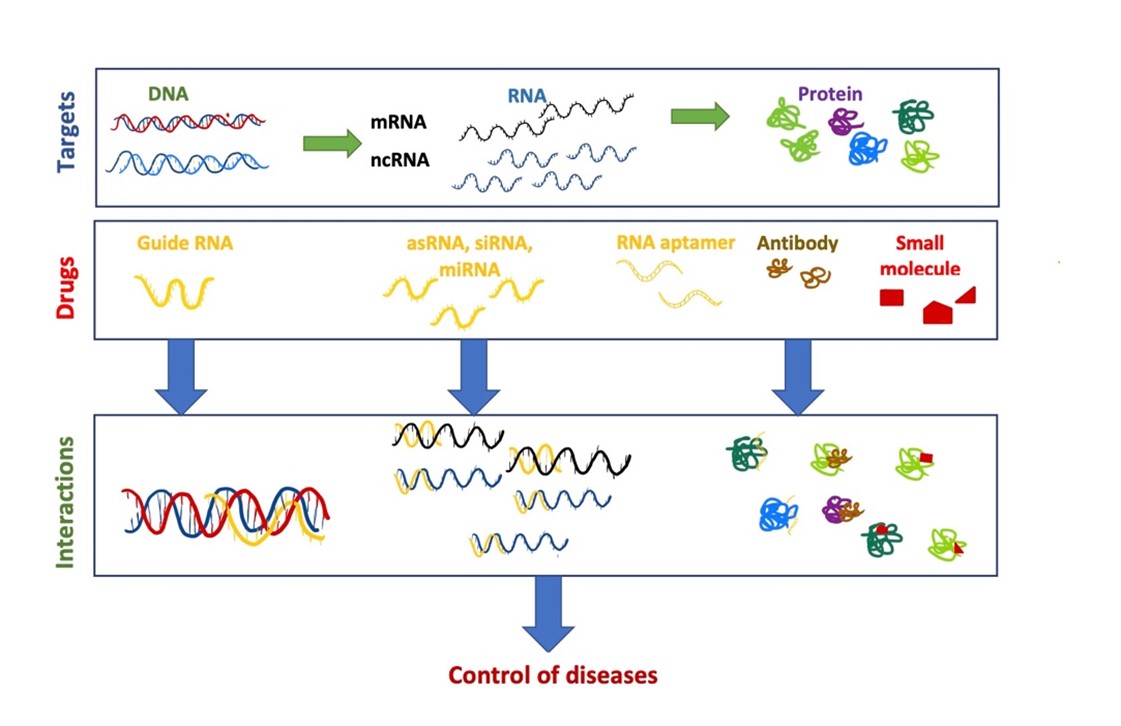
RNA therapeutics are types of medications made from RNA (ribonucleic acid). The types based on mRNA (messenger RNA), asRNA (antisense RNA), RNAi (interference) and RNA aptamers are the most widely used (figure 1) (Yu et al., 2019). RNA therapy provides or modifies RNA to patients’ cells, and therefore has the potential to treat a variety of diseases. Cardiovascular disease, cancer and hemophilia are three diseases that can be treated by RNA therapy (DeWeerdt, 2019).
|
Figure 1 Function of RNA. |
Messenger RNA-based therapy is particularly useful in vaccine development, since it is based on inducing synthesis of proteins within cells. Only recently, the mRNA vaccines have been developed towards the COVID-19 pandemic. Antisense RNA, which is complementary to coding mRNA, and RNAi are used to prevent the mRNA from being used in protein translation. RNAi is also discovered as a method to inhibit the expression of some individual genes by double-stranded RNA. This will probably be possible to use as treatment for diseases like cancer in the future (Guo et al., 2013).
Most RNA therapies can be sorted into one of three broad categories: those that target nucleic acids (either DNA or RNA), those that target proteins, and those that encode proteins (Peters, 2018).
Target nucleic acid: single-stranded antisense oligonucleotides (ASOs) and double-stranded molecules known as RNA interference (RNAi)
Target proteins: RNA aptamer – it binds to a specific site on a specific protein to modify its function
To develop personalized cancer vaccines, RNA therapies that use mRNAs are used. Researchers are now exploring whether this mRNA treatment can be used to encode proteins. It could then work as a protein-replacement for i.e., hemophilia, which is a blood-clotting disease (‘RNA Therapeutics’, 2021).
Effects of RNA therapy
One of the greatest advantages of RNA therapy is the ability to target molecules known as undruggable. Since RNA drugs can bind specifically to nearly all types of RNAs, cellular molecules that have RNA can be targeted, whether they are in a stage during production or as a final form. Another advantage of this form of treatment is it long-lasting effects. This was proven in a recent clinical trial where the expression target gene PCSK9 was suppressed with great effect and the decreased level of cholesterol was maintained even after 6 months after the treatment. The long-lasting effects when using iRNA treatment is considered beneficial for patients which may not benefit from frequent treatments.
RNA therapy that targets proteins need to use a special molecule named as RNA aptamer. This molecule is made to bind to a specific site on a specific protein to be able to adjust its function. An example for this is pegaptanib, a treatment for an age-related macular degeneration where blood vessels penetrate into the retina and causes vision to decline. Pegaptanib are able to bind and block the function of the protein that causes the vascular endothelial growth factor, which will lead to growth reduction and permeability of the blood vessels in the eye. RNA aptamers are still being studied, and scientists hope that the rapid action and reversibility could aid during surgery and emergency medicine in the future.
Apic Bio in Cambridge, Massachusetts, uses RNAi in an approach called silence and replace. This method uses RNAi component to silence a harmful gene and mRNA components are used as a replacer and encodes a normal version of the corresponding mutated protein. The company are currently doing preclinical work on hybrid drugs aiming on inherited lung and liver diseases and hereditary sclerosis, which is a degenerative neurological condition.
Despite all of the promising effects of RNA based therapy there is one big barrier the scientists are facing. It has proven difficult to introduce the RNA to the correct place in the correct cells. To solve this problem of intracellular delivery several delivery strategies are being utilized. Lipid nanoparticles are now undergoing clinical trials for this purpose. They have proven useful as they protect the RNA and increases their half-time, as well as increasing the efficiency of endocytosis into the cells. An additional strategy is the conjugate. As the conjugate binds to specific receptors on the cell surface, it can be utilized to deliver the covalently bound cell surface receptor, the RNA-conjugate complex is taken into the cells through receptor-mediated endocytosis. However, there are still no good strategies for delivering RNA drugs to tissues other than the liver efficiently, therefore the most essential studies for the future is to develop efficient ways of delivering drugs to other tissues which have less relation to the liver (DeWeerdt, 2019)
Immunological background
RNA interference (RNAi) technology has made it possible to interfere with the innate and adaptive immune systems by modifying the mechanisms that regulate the functions of the cells. By performing therapy on the RNA instead of the DNA itself, modifications are possible without changing the actual genes of the patient, like in gene technology. Instead, it is a method to activate or inhibit certain processes/ immune responses in the body. By changing the ribonucleic acids of mRNA to code for a protein of desired function, immune responses in the body can be stimulated or suppressed. Compared to placing protein or DNA into a cell, using RNA molecules directly is advantageous in therapy. When using DNA, long sequences must be delivered into the nucleus. While RNA molecules can be produced both chemically and in vitro, which means it only must be delivered to the cytosol. Therefore, RNA therapy is of immunological importance, as the technique may be used to regulate the immune response for clinical purposes, e.g., Vaccines. It can also be used as RNAi (RNA interference), where it suppresses a gene or only the mRNA variant of the gene causing the disease.
The immune system is divided into two parts: the innate and the adaptive. The cells of the innate immune system (macrophages, neutrophils and natural killer cells) provide an initial immune response. On the contrary, the adaptive immune system (B- and T-cells) produce antibodies against the pathogens or control infected cells through a cell-mediated process which is highly antigen specific. RNA interference has contributed to a greater understanding of immune system disorders and mechanisms by which pathogens are able to evade the hosts immune system in a clinical setting.
Neutrophils are found circulating in the blood, and is an important component of the innate immune system. When an infection occurs, the neutrophils are stimulated and migrate to the site of infection in a process called chemotaxis. The neutrophils help fight infection through recognition, phagocytosis and killing of the pathogens, or by realizing factors that stimulate immune response against the pathogens. Studies have shown that RNAi may be used to regulate the migration of neutrophils to the site of an inflammation, representing a potential strategy for the prevention of tissue injury by inappropriate inflammation.
Macrophages are the primary components of the innate immune response. They perform phagocytic activity and contribute to the inflammatory process, but also develop into antigen presenting cells and initiate the adaptive immunity. RNA therapy has been used to characterize the function of the macrophages, and record their behavior for clinical purposes. RNA has been used to investigate the signal pathways regulation macrophages activation and macrophage-induced inflammation.
As known, DNA is transcribed into mRNA and thereafter translated into proteins. Proteins play important roles in regulating vital processes. RNA therapy works by disturbing certain parts of this process (Livingstone, 2015).
- Degradation of mRNA complex: RNA based drugs have the ability to use intracellular RNase HI or form RISCs to cut and discard of certain mRNAs to achieve the goal.
- Regulating the splicing process of mRNA: RNA drugs can both enhance and inhibit certain RNA splicing. This is achieved by combination to mRNA splicing sites, in order that they can regulate the gene expression.
- Regulating protein expression: Fake transcription factors can be induced to target mRNAs and block translation. This can reduce the production of disease-causing proteins, or even increase the translation of synthetic mRNA and by that enhance the production of therapeutic proteins.
- miRNA mimics: The miRNA works similarly to natural miRNAs. The miRNAs are responsible to regulate necessary developmental functions and pathways for maintaining cell recognition and by this better the function of endogenous miRNA and decreasing intracellular proteins. These methods can potentially help in cancer therapy by reinstituting broken or disrupted miRNA maturation mechanisms and by enhancing expression of specific tumour suppressor miRNAs.
- Anti-miRNA oligonucleotides: miRNA inhibitors are synthetically made ASOs that have the ability to weaken the silencing effect of endogenous miRNAs by specifically binding to the active chains and increase the protein expression.
- miRNA competitive antagonists: miRNA competitive agonists block RISCs from pairing with targeted mRNAs by “protecting” miRNA sites of binding amongst them, and by this regulating the expression of certain genes.
Noncoding RNAs have important functions in physiological and pathophysiological cases. According to studies, modulating noncoding RNAs can be an option for therapeutics. microRNAs and long noncoding RNAs are mainly what the noncoding RNAs compromise. The two kinds have different functions. MicroRNAs can bind the 3’untranslated region of a mRNA, and then block protein translation, or induce its degradation. This makes it useful for regulation of gene expression pattern. Long noncoding RNAs have many different functions. Mainly it works as an epigenetic regulator, molecular scaffold or a decoy. Both types of the noncoding RNAs might be helpful for treatment of cardiovascular diseases, including heart failure, fibrosis, acute myocardial infarction, and atherosclerosis (Lucas et al., n.d.).
For cardiac fibrosis, several miRNAs are implicated to either reduce the extent of injury (i.e., by targeting angiogenesis or cardiomyocytes death after heart infarction) or interfering directly with the fibrotic response itself. An effective therapy against heart failure might be the modifying of intracellular calcium levels. That is done by improving the contractility of cardiomyocytes (Mao et al., 2007).
Tumours occur when the cell cycle turns uncontrolled. Therefore, the first attempts on RNA therapy for treating tumours was based on inhibiting the unlimited proliferation of tumours on RNA level. Ali et al. had a breakthrough when they discovered that a few long non-coding RNAs that was very frequent during the cell division in mice suffering from lung cancer could be turned off by injecting nucleic acid-modified antisense oligonucleotides. This procedure contributed to curing 40-50% of lung tumours in the investigated mice (Mao et al., 2007).
The KRAS gene is one of the most frequent mutated genes in human cancer medicine. If the KRAS gene mutates, it causes uncontrolled cell division. KRAS was previously classified as an undruggable gene. After inducing mice with the KRAS mutated gene Pecot et al. injected them with siRNA with a nanoliposomal delivery platform, and the KRAS oncogene was silenced successfully. This breakthrough made both pERK and pMEK relevant in cancer treatment studies as they did not just restrain the growth of tumours, but also controlled the spread (Liang et al., 2020).
Conclusion
RNA therapy is an important factor in the study of immunology as well as being of great clinical importance. Medications derived from RNA therapeutics has pathological significance, as RNA therapy can be used to cure several diseases, e.g., cardiovascular disease or cancer. The messenger RNA is used in the development of vaccines, and has been highly significant in the development of the Covid-19 vaccines recently. The great difference between DNA therapy and RNA therapy lies in the ability to perform the therapy on the RNA instead of the DNA itself, leaving the genes of the patient unchanged. It advantageous by its ability to target molecules during production or in the final from, as well as having a long-lasting effect. The main issue facing scientists today regarding RNA based therapy is the difficulty to deliver the RNA to the correct place in the correct cell.
Citations:
Chen, H., Ye, F., & Guo, G. (2019). Revolutionizing immunology with single-cell RNA sequencing. Cellular and Molecular Immunology, 16(3), 242–249. https://doi.org/10.1038/s41423-019-0214-4
DeWeerdt, S. (2019). RNA therapies explained. Nature, 574(7778), S2–S3. https://doi.org/10.1038/d41586-019-03068-4
Guo, W., Chen, W., Yu, W., Huang, W., & Deng, W. (2013). Small interfering RNA-based molecular therapy of cancers. Chinese Journal of Cancer, 32(9), 488–493. https://doi.org/10.5732/cjc.012.10280
Liang, X., Li, D., Leng, S., & Zhu, X. (2020). RNA-based pharmacotherapy for tumors: From bench to clinic and back. Biomedicine & Pharmacotherapy, 125, 109997. https://doi.org/10.1016/j.biopha.2020.109997
Livingstone, M. (2015, September 30). RNA Therapy and the Innate Immune Response | tebu-bio’s blog. https://www.tebu-bio.com/blog/2015/09/30/rna-therapy-and-the-innate-immune-response/
Lucas, T., Bonauer, A., & Dimmeler, S. (n.d.). RNA Therapeutics in Cardiovascular Disease | Circulation Research. Retrieved 29 April 2021, from https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.117.311311?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed&
Mao, C.-P., Lin, Y.-Y., Hung, C.-F., & Wu, T.-C. (2007). Immunological research using RNA interference technology. Immunology, 121(3), 295–307. https://doi.org/10.1111/j.1365-2567.2007.02599.x
Peters, J. (2018, August 31). Haven’t heard of RNA therapy yet? You will. Massive Science. https://massivesci.com/articles/rna-therapy-treatments/
Other materials used:
RNA therapeutics. (2021). In Wikipedia. https://en.wikipedia.org/w/index.php?title=RNA_therapeutics&oldid=1018498133
Figure:
Yu, A.-M., Jian, C., Yu, A. H., & Tu, M.-J. (2019). RNA therapy: Are we using the right molecules? Pharmacology & Therapeutics, 196, 91–104. https://doi.org/10.1016/j.pharmthera.2018.11.011