Immunometabolism
by Ruby Reinefjord
Supervisor: Dr. István Tóth
Contents
Introduction
Two of the most crucial prerequisites for the survival of living organisms are functioning metabolism and immune system. The immune system is known for its role in recognition of and defence against viruses and other pathogens whilst metabolism is the motor of the body, providing energy to carry out various functions of the organism through chemical processes. Metabolism and the immune system have previously been investigated independently of each other since there has been no obvious relationship between the two. However, the interface between them is now an emerging field of study, fittingly named “immunometabolism”. Immunometabolism can be considered from two perspectives: 1) the role of metabolic pathways in immune cells and the effect on broader immunity, or 2) the role of immune cells in metabolism in organs and the effects on whole organism metabolism (Pearce and Pearce, 2013). The latter of which will be discussed in this essay when investigating how immune responses can cause malfunctioning of the body.
The physiological effects of immunometabolism
|
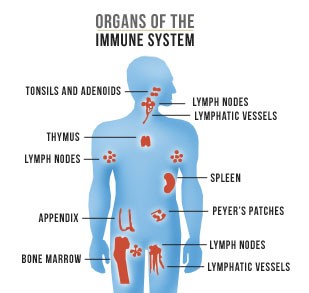
The main organs of the immune system are divided into two groups: The primary (central) organs including the thymus, bursa, embryonic liver and bone marrow, and the secondary (peripheral) organs consisting of the spleen, lymph nodes, mucosa associated lymph tissue and gut associated lymph tissue. These organs are the site of production and maturation of different immune cells that either belong to the innate- or the adaptive immune systems. Cells of the innate immune system are non-antigen specific and activated by toll-like receptors which results in immediate response whilst cells of the adaptive immune system actively respond to foreign antigens but after a latency period. Together, these two systems contain an almost endless number of immune cells, all with their own functions and roles in the complex defense-system of the body.
As mentioned in the introduction, the studies made on immunometabolism covers everything from the finest biochemical details to the more physiology-related effects on organs and the living organism as a whole. Among a huge number of factors studied, three particular topics are recurring in several studies and seems to have a greater relevance than others when investigating the physiological effects of immunometabolism. These are: effect on the development of diseases, effects on growth and the role of feed and additives. In all three cases, innate immunity seems to have the more significant role out of the two immune systems.
Diseases caused by immunometabolic related disorders
The earliest studies made on immunometabolism mainly involved human health concerns related to obesity, diabetes, and other metabolic disorders where it could be proven that excessive fat deposition can lead to an innate immune inflammatory response, i.e. a non-antigen specific reaction. Chronic low-grade inflammation has then further been linked to metabolic diseases, such as type 2 diabetes, atherosclerosis and fatty liver disease (Ferrante, 2013).
More specifically, it all started about 20 years ago when the Turkish professor Gokhan Hotamisligil (Hotamisligil et al. 1993) found a connection between obesity and sudden appearance of the prototypical inflammatory protein TNF-α (tumor necrosis factor-α) in adipose tissue of both rodents and humans. Further investigations indicated that TNF-α mediates obesity-associated insulin resistance which could be one of the reasons for type 2 diabetes to occur. Later, other groups of scientists have built on these initial observations to demonstrate that obesity activates the expression of not only TNF- α, but more than 50 different inflammatory molecules in fat (Ferrante, 2013). Most of these inflammatory molecules were demonstrated to be derived from adipose tissue macrophages which had the scientists quickly convinced that typical rodent and human adipose tissues contain macrophages at levels proportionate to adiposity. All these statements added together now lays the foundation for clearly representing a complex conversation between stressed adipocytes and the immune system.
Even though adipose tissue is the most obviously affected area, obesity has also been shown to change immune cell populations in the liver, bone marrow, key regulator areas of the hypothalamus, and the circulation (Ferrante, 2013). Hence, diseases such as previously mentioned atherosclerosis and nonalcoholic fatty liver disease can also occur due to overweight.
As years have passed, work by many laboratories has revealed that obesity induces a complex and broad immune response involving nearly all innate and adaptive immune cell types (Ferrante, 2013). There are still some disagreements between scientists about what the initiating factor is and it requires further investigation. Some studies suggest that infiltration of neutrophils is the initiating immune event, others say that it is the reduction in regulator T cells, while a third group of studies implicate that rapid changes in macrophages is the true reason for the immune response to appear. It is, either way, important to mention that metabolic regulation of the immune system is not exclusively limited to obesity. Fasting, cold challenge, weight loss, and acute lipolysis all increase the macrophage number and function in adipose tissue. This is quite logical considering both starvation and reduced leptin concentrations has inhibitory effects on immune function and effector T-cells which is the reason for scientists believing that the immune system monitors and responds to the metabolic state of tissues and therefore the whole organism. (Ferrante, 2013)
Immunometabolic effects on growth
|
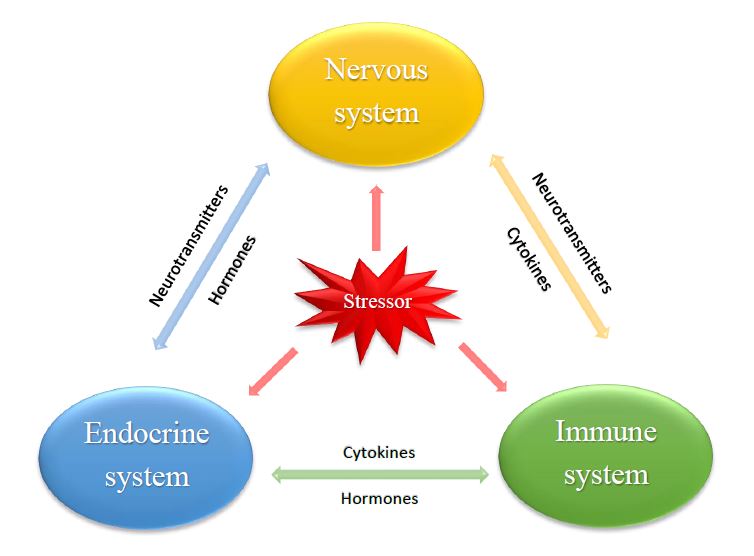
In addition to diseases caused by immunometabolical disorders, long lasting innate immune responses has also been shown to affect growth. Research combining the study of immunity and metabolism in animal production investigated how maintaining an active immune response over a longer period affected energy levels and the transfer of energy from the growth process to the immune system (Klasing and Korver, 1997). The conclusion was that animals that initiate inflammatory response during their growth period are more likely to grow slower as well as to have an impaired feed conversion. The reason for this is the release of pro-inflammatory cytokines which is a natural part of the immune system’s response to an infectious stimulus. Cytokines are a broad category of small proteins which are synthesized and secreted mainly by immune cells, like B- and T-lymphocytes, but also some non-immune cells such as endothelial cells and fibroblasts. Cytokines have an important role in both autocrine-, paracrine- and endocrine signaling as immunomodulating agents. They also share a special relationship with another category of signaling molecules, peptide hormones. These two types of molecules, belonging to the immune- and endocrine system respectively, together with the nervous system make up a complex arrangement in the body where all factors are dependent of each other forming a “neuro-immuno-endocrine-relationship”, as can be seen in Figure 2. The immune system is innervated and shares a set of hormone and cytokine receptors with the endocrine system making it possible for the two systems to regulate each another. In other words, the immune system produces and responds to neuroendocrine peptide hormones and neurotransmitters whilst the nervous system produces and responds to the immune system’s cytokines.
Release of certain types of cytokines, such as interleukin-1 (IL-1) and previously mentioned tumor necrosis factor-α (TNFα), mediates a so called “stress-response” that induces specific behavioral, cellular, and metabolic changes in the body. These physiological changes alter the partitioning of nutrients away from growth and toward processes that instead support the immune and inflammatory responses. Cytokines induce distinct endocrine changes that further impair growth, including increased glucocorticoids and decreased insulin like growth factor-I by acting directly on most tissues of the body through specific receptors.
In a study made on growth reduction in young chicks (Klasing et al. 1987), changes of both the liver and skeletal muscles could be observed as IL-1 and TNFα act synergistically to increase the rate of protein degradation in chick skeletal muscle. IL-1 induces the release of corticosterone that decreases the rate of skeletal muscle protein synthesis and further slows down skeletal muscle accretion. However, due to the increased secretion of acute-phase proteins by pro-inflammatory cytokines and corticosterone, protein synthesis of the liver will conversely be elevated. This means that an inflammatory response give rise to a change in both the rate and the composition of growth in a manner that varies directly with the amount of pro-inflammatory cytokines released. (Klasing and Korver, 1997)
To solve the problem of growth reduction due to the body’s automatic innate immune response it has been discussed whether certain types of growth promoting antibiotics could have a positive counteracting effect. Especially in human medicine, it has been shown that less incidence of disease as infants results in greater growth and ultimate height in adulthood. One of the benefits of prescribing antibiotics is its anti-inflammatory effects; and since inflammation is one of the reasons that growth may diminish at an early age, the theory was thought to be promising for future studies. However, societal concern and government regulations increasingly press for restricting the use of antibiotics as antimicrobial growth promoters as they may cause changes of the intestinal wall and therefore disturb the rather complex and dynamic microflora-system of the intestines. The further search for alternatives is for that reason aimed at nonantibiotic compounds that still has similar effects on the inflammatory system as the antibiotics. (Niewold, 2007)
The significance of feed-intake and additives
Since obesity, as previously discussed, is thought to have certain connections to inflammatory response, a third aspect must be taken into consideration: Feed-intake. In the animal science field, feed-induced inflammation has been a concern for years and the study seems to be more relevant than ever as the problem of overweight is constantly increasing in both the human and animal populations. According to the Association for Pet Obesity Prevention, an estimated 53.8% of US dogs and 58,2% of US cats were overweight or obese in the year of 2015 (Association for Pet Obesity Prevention, 2016).
Regarding animals, certain feed ingredients are more likely to initiate an inflammatory gut-response, examples are non-digestible components of wheat and rye in chickens (Teirlynck et al. 2009), soybean meal in fish (Urán et al. 2008) and even overall excessive feed-intake has shown to initiate changes in the immune response (Klasing, 1988). The main solution has previously been to add enzymes to the food to break down certain indigestible and/or inflammatory feed components in the gut, with the aim to reduce immune response and redirect this energy to growth. A newer feeding strategy involves trying to find natural additives that enhance the animal’s resistance to disease, either by influencing the host immune response or the gut microbiota. However, evaluation of these feed additives must be exercised with caution since it is important to determine efficacy and understand the mechanism of action precisely.
One scenario where this new strategy could come in handy is for example the transition period immediately before and after calving in dairy cattle, as this is an important immunometabolic period. A dairy cow’s immune functions are impaired during the transition period, as the mobilization of lipids causes susceptibility to both metabolic and infectious disease. This results in an increase in fatty acids in the blood which can lead to uncontrolled inflammation and oxidative stress. This dysfunction in the inflammatory response, due to the free fatty acid increase, is the link between metabolism and immunity during the calving period. (Sordillo and Raphael, 2013)
In another production species, poultry, there has been a greater amount of research done on the use of pre- and probiotic feed ingredients as a counteracting factor to nutrition’s effects on immunity (Hume, 2011). The immunometabolic link between stress or disease and production issues in poultry is still described with limited literature but so far signs of both lameness (Kogut, 2013) and muscle fat deposition (Arsenault et al. 2013) have been linked to immunometabolism problems.
Conclusion
Immunometabolism is clearly an interesting and relevant field of study as it has proven to be linked to several physiological issues of the living organism that could possibly be prevented with further research. Regarding obesity, the study made in the US from 2015 provides yet another reason to reduce obesity in the animal population which for example has been shown to be particularly proliferated in cats and dogs from the united states of america. Since long lasting immune responses caused by excessive fat deposition has shown to give rise to several chronic diseases, a quick solution focused on anti-inflammatory preparations might be beneficial. The natural fat-reducing methods are usually increased exercise or diets but these can be both time-consuming and give varied results depending on the individual. However, the use of anti-inflammatory preparations should probably be used as a temporary supplement to exercise only, since it is important to not only slow down the development of an inflammatory response but to get rid of the actual source of the problem by burning excessive fat.
Moreover, integrating metabolism and immunity also provides a new research avenue for the ultimate goal of increasing growth and improve animal production without having a negative impact on animal health and immunity.
Lastly, whether growth-reduction of animals in the production industry should be remedied with growth-promoting antibiotics or pre- and probiotic feed ingredients is still arguable. The pros and cons of the two at this stage are not convincing enough to make one method a clear winner but it is however quite obvious that natural feed-additives ought to be the more sustainable solution considering the potential side-effects of regular antibiotic consumption.
References
Arsenault, R. J.; Napper, S.; Kogut, M. H. (2013): Salmonella enterica Typhimurium infection causes metabolic changes in chicken muscle involving AMPK, fatty acid and insulin/mTOR signaling. Veterinary Research 44(35). https://veterinaryresearch.biomedcentral.com/articles/10.1186/1297-9716-44-35
Ferrante, A. W. JR. (2013): Macrophages, fat, and the emergence of immunometabolism. J Clin Invest. 123(12): 4992–4993. http://www.jci.org/articles/view/73658
Hotamisligil, G. S.; Shargill, N. S.; Spiegelman, B. M. (1993): Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 259(5091): 87-91. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC295872/pdf/jcinvest00026-0467.pdf https://www.ncbi.nlm.nih.gov/pubmed/7678183
Hume, M. E. (2011): Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics. Poult Sci. 90(11): 2663-2669. https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2010-01030
Klasing, K. C. (1988): Influence of Acute Feed Deprivation or Excess Feed Intake on Immunocompetence of Broiler Chicks. Poult Sci. 67(4): 626-634. https://academic.oup.com/ps/article-abstract/67/4/626/1529508/Influence-of-Acute-Feed-Deprivation-or-Excess-Feed?redirectedFrom=fulltext
Klasing, K. C.; Korver, D. R. (1997): Leukocytic Cytokines Regulate Growth Rate and Composition Following Activation of the Immune System. ANIMALSCI. 75(2): 58-67. https://dl.sciencesocieties.org/publications/jas/abstracts/75/Supplement_2/JAN0750S20058?access=0&view=pdf
Klasing, K. C.; Laurin, D. E.; Peng, R. K.; Fry, D. M. (1987): Immunologically mediated growth depression in chicks: influence of feed intake, corticosterone and interleukin-1. J Nutr. 117(9): 1629-1637. https://www.ncbi.nlm.nih.gov/pubmed/2443625
Kogut, M. H. (2013): The gut microbiota and host innate immunity: Regulators of host metabolism and metabolic diseases in poultry? J Appl Poult Res. 22(3): 637-646. https://academic.oup.com/japr/article-lookup/doi/10.3382/japr.2013-00741
Niewold, A. T. (2007): The Nonantibiotic Anti-Inflammatory Effect of Antimicrobial Growth Promoters, the Real Mode of Action? A Hypothesis. Poult Sci. 86(4): 605-609. https://academic.oup.com/ps/article-lookup/doi/10.1093/ps/86.4.605
Pearce, E. L.; Pearce, E. J. (2013): Metabolic Pathways in Immune Cell Activation and Quiescence. 38(4): 633-643. http://www.cell.com/immunity/fulltext/S1074-7613(13)00158-1
Sordillo, L. M.; Raphael, W. (2013): Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Veterinary Clinics of North America: Food Animal Practice. 29(2): 267-278. http://www.sciencedirect.com/science/article/pii/S0749072013000297
Teirlynck, E.; Bjerrum, L.; Eeckhaut, V.; Huygebaert, G.; Pasmans, F.; Haesebrouck, F.; Dewulf, J.; Ducatelle, R.; Van Immerseel, F. (2009): The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. 102(10): 1453-1461. https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/cereal-type-in-feed-influences-gut-wall-morphology-and-intestinal-immune-cell-infiltration-in-broiler-chickens/87489ABB305C708BFC46AE05F07B3C9A
Urán P. A.; Gonçalves A. A.; Taverne-Thiele J. J.; Schrama J. W.; Verreth J. A. J.; Rombout J. H. W. M. (2008): Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish & Shellfish Immunology. 25(6): 751-760. http://www.sciencedirect.com/science/article/pii/S1050464808000569
Webpages
Association for Pet Obesity Prevention (2016): 2015 Obesity Facts & Risks. (Accessed 21 April 2017). http://petobesityprevention.org/pet-obesity-fact-risks/
Picture references
Figure 1:
United States Department of Health and Human Services (2015): Organs of the Immune System. Wikimedia Commons: “As a work of the U.S. federal government, the image is in the public domain.” https://commons.wikimedia.org/wiki/File:Organs_of_the_Immune_System_by_AIDS.gov.jpg
Figure 2:
Reinefjord, R. (2017): Interactions between the immune-, endocrine- and nervous systems. © Ruby Reinefjord.