The pharmacology of metformin, the new anti-aging drug
Contents
-
The pharmacology of metformin, the new anti-aging drug
- Introduction
- The diverse positive effects of metformin on the life span and health span
-
Mechanism of action
- Therapeutical dosage recommandations
- Pharmokinetics of metformin
-
Main actions of metformin
- Most recently accepted scientific model for the global mechanim of action of metformin
- Action on AMPK in the liver
- Action linked to anti-hyperglycemia effect
- Action linked to anti-oxidant effect
- Action linked to anti-cancer effect
- Action on mitochondria complex 1
- Action on vascular endothelium
- Action on female reproductive system
- Pharmacovigilance of Metformin
- Conclusion
- References
Introduction
Metformin is currently the most prescribed and used drug in the world against type -2 diabetes or non-insulin-dependent diabetes mellitus (NIDDM) (Dunn and Peters, 1995).
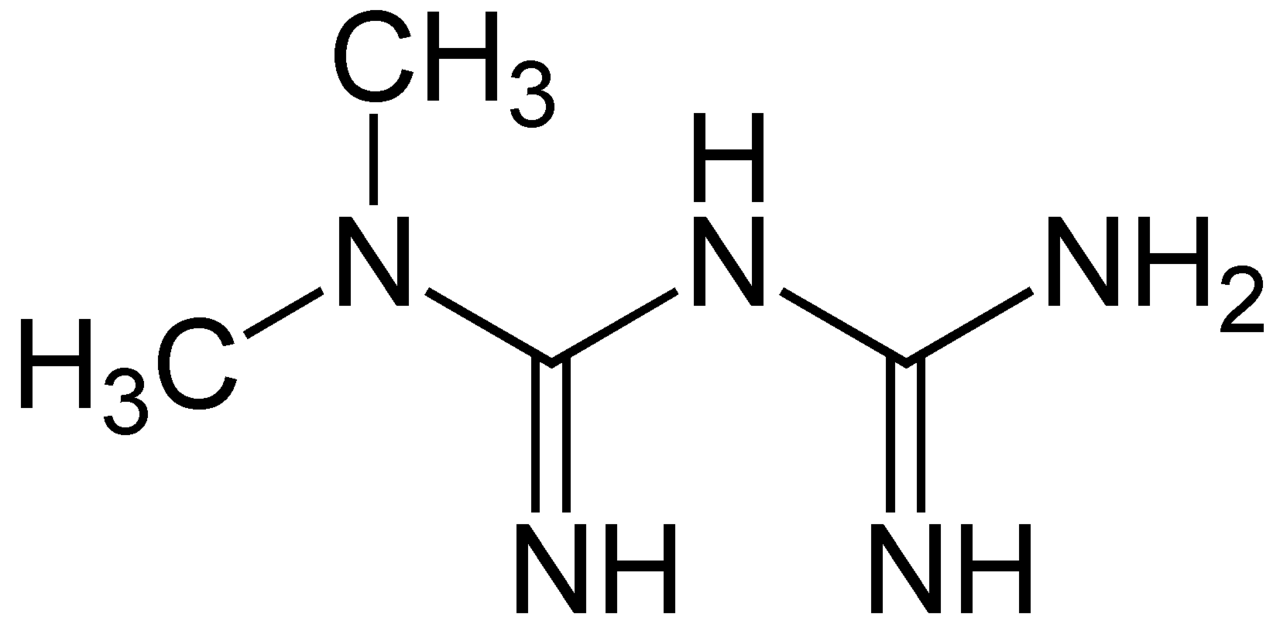
Metformin is a derivative of biguanidin (Figure 1). It has been used to replace another biguanidin molecule called phenformin which causes a higher risk of mortality (Dunn and Peters, 1995). As a safer and relatively cheap drug, its use has been exponentially increased from 1980s (Vecchio et al, 2014). Now, it is known as the first line of prescription. It is marketed under the trade name Glucophage or its generics. Metformin oral tablet is the most common drug form (Figure 2) (Dunn and Peters, 1995).
In addition to its anti-diabetic effects, other positive effects were reported by different studies on laboratory animals (Barzilai et al, 2016). It could reduce the effects of age and increase the lifespan . These properties are very interesting for the scientific community. So, a new project called “Targeting Aging with Metformin” (TAME) is in progress (Barzilai et al, 2016). Its goal is to study the link between metformin and its potential anti-aging effects on humans.
In this essay, the common anti-aging effects are described. Then, the current consensed mechanism of action of metformin is presented. Finally, the pharmacovigilance including the most observed side effects of this drug is reported.
|
|
The diverse positive effects of metformin on the life span and health span
Metformin and its effects on lifespan of different animal species
Positive dose-dependant effects of Metformin on the lifespan of worms
Onken and Driscoll (2010) studied the effects of metformin therapy on nematodes species C elegans. The concentration of 0.00001 to 1.0 mg/l has been applied starting from the larval stage of the nematode untill its whole life . Metformin added at 50 mM dose was shown to increase only the mean lifespan (not the maximum lifespan). A supplementation of concentration of 10 or 100 nM doses didn’t had any significant impact on the lifespan of the C elegans. Anisimov (2013) similarly reported that metformin therapy at doses 25nM increases mean lifespan by 18%, at 50 nM the increase is by 36% and at 100 mM by 3%.
Thanks to these studies, it was shown that metformin given at a certain concentration prolongs nematode health span, extends their mean lifespan and prolongs their youthful locomotor ability.
Effects of metformin on the lifespan of different mice models
Postive effects of metformin on female mice with tumors predisposition
Studies were made on female transgenic HER-2/neu group of mice which is a mice model expressing tumors (indeed this strain of mice mostly develop spontaneous mammary tumors, pulmonary and lymph node metastases). While comparing HER-2/neu group to a control group it has been shown that the supplementation of metformin slowed down the age-related rise of blood glucose, the triglyceride and cholesterol levels and the age-related irregularity in the estrous cycle. Furthermore, it reduced the level of β-lipoproteins. It extended the mean lifespan by 4-8% and the maximum lifespan by 1 month. In addition, it normalized the expression of cytolytic granzyme B and perforin gene in malignant mammary tumors (Anisimov, 2010).
Age-dependant metformin effects on SHR female mice
A study on female SHR mice (acronym for Spontaneously Hypertensive Rat, which are rat with hypertension and used to study cardiovascular diseases) showed that a supplementation with metformin increased the mean lifespan of the survivors by 20% and their maximum lifespan by 10.3% (Anisimov, 2013). Metformin treatment delayed the age-related irregularity of the estrous function. However, no effect was observed on the following points : serum level of cholesterol, triglycerides, glucose, insulin and carcinogenesis. Another study based on different set of age of SHR mice showed that Metformin given at age 3, 9 and 15 months increased the mean lifespan by respectively 14%, 6% and 0% (Anisimov, 2010).
Sex-dependant effect of metformin on 129/Sv mice and trangenic mice with Huntington disease (HD)
In 129/Sv mice as animal model test, a supplementation of metformin of 100 mg/kg induced a modification of their food intake but didn't change their body weight. Applied on male 129/Sv mice, a decrease of the lifespan of 13.4% and no decrease of spontaneous tumor development were observed. On the contrary, an increase of 4.4% of the mean lifespan and a decrease of the spontaneous tumor development were reported on female 129/Sv mice (Anisimov, 2010). Other studies were made on transgenic mice with Huntingon disease, which is a neurodegenerative disease that leads to striatal degeneration and severe movement disorder. Metformin treatment increased the lifespan on male mice by 20.1%. However, no increase were observed on female mice receiving the same treatment (Anisimov, 2013).
Dosage-dependant effect of Metformin on male C57BL/6 and B6C3F1 mice
Male C57BL/6 mice (which is the most used laboratory rodent) supplemented by 0.1% of Metformin on diet ad libitum had an increase of 5.83% of their lifespan. A supplementation of 1% of Metformin leads to a decrease of 14.4% of their lifespan (Ani et al, 2013). The same experiment on B6C3F1 mice resulted by an increase of the lifespan by 4.15% on a ad libitum diet of 0.1% of metformin (Anisimov, 2013). The anti-ageing effects of metformin depends on the dosage and on the strain of the mice.
General effects of Metformin observed on mice
In all the previously mentioned studies, some particular beneficial effects were pointed out such as improved physical performance, delayed metabolic syndrome such as improvement of glucose tolerance, increase of the insulin sensitivity, a decrease of the LDL ( Low Density Lipoprotein) and cholesterol level. These positive effects depends on the stain of the mice, on the sex, on the onset of the treatment and on the dosage of metformin.
No significant or toxic effect on the increase of lifespan of F344 rats and Drosophila
6 months old male F344 rats were divided in 4 groups : a control group, a calorie restricted group, a metformin supplemented group and pair fed to metformin (Anisimov, 2010). All group presented no significant differences concerning their mean lifespan and the mean age of the last surviving 10%. The researchers suggest that the effect of metformin is similar to the effect of calorie restriction. In a similar way, independent studies on male and female Drosophila have shown that metformin supplemented in doses 0.4, 0.8, or 0.16 mg/ml doesn’t influence their mortality rate (Anisimov, 2010). No significant effect on their survival has been shown. A significant decrease of their survival were even observed at the maximal dose. Consequently, metformin may have no significant or a toxic effect on the lifespan on some animal species like F344rats or Drosophilia.
Conclusion on the effect of Metformin on laboratory animals
These study on animals showed that Metformin therapy has generally a positive dose-dependant effect on lifespan and healthspan in mice and worms. Indeed, metformin can be toxic above a species-dependant level. Metformin therapeutic dosage is species and strain specific.
Metformin therapy reduces the risk of age-related cancer
Study of the effects of metformin on ovarian cancer patients with diabetes
Metformin reduce the diabete type 2 and consequently it reduces the risk of cancer. A study on 568 consecutive patients, newly diagnosed with ovarian cancer including patient with ovarian, fallopian or peritoneal cancer and excluding patients with type 1 diabetes, incomplete medical records and patient with any other cancer before their ovarian cancer diagnosis. 48 (8.5%)of these 568 patients with type 2 diabetes received a continuous treatment of metformin, 34 (5.9%) patients with type 2 diabetes were part of the diabetic control group(no metformin treatment), 22 (3.9%) patients with type 2 diabetes received a discontinued metformin treatment, and 464 (81.7%) ovarian cancer patients were part of nondiabetic controls. The results showed that the group taking continuous metformin treatment had a longer stabilization of the tumors (progression-free survival) and a better overall survival (OS) compared with the 3 others groups (P = 0.001). Furthermore, patients taking continuously Metformin had a lower risk for disease relapse death [ P= 0< .01] and disease-related death (P= .03) ( Wang et al, 2017). Consequently it’s required that the Metformin treatment has to be continuous in order to have beneficial effects such as a reduction of the risk of disease recurrence and a reduction of risk of death in ovarian cancer patients having diabetes.
2.2.2.2 Effects of metformin on cancer occurence and mortality
According to statiscal studies (Table 1), metformin treatment is associated to the reduction of the risk of cancers. It significantly reduces the occurence of the most frequent and severe age-related cancers such as colorectal, liver, pancreatic, stomach or oesophagus cancer. Consequently, metformin is a very interesting potential anti-tumor drug.
Type of cancer/ other Pathology |
Number of studies |
Total numbers of studied patients |
Effects of Metformin treatment |
Statistical results |
Ovarian cancer patients with diabetes |
1 |
568 |
(1) stabilization of the tumor and better overall survival , (2) lower risk for disease, relapse death and lower risk of disease-related death |
(1)** (2)* |
Cancer mortality |
11 |
28 671 |
significant reduction of the risk of death related to cancer |
*** |
Malignancy |
25 |
579 621 |
significant reduction of the risk of any malignancy |
** |
Colorectal cancer |
12 |
871 365 |
risk of colorectal cancer decreased |
*** |
Liver cancer |
8 |
312 742 |
significant reduction of the risk of liver cancer |
*** |
Pancreatic cancer |
9 |
847,248 |
reduction by 44% of the risk of pancreatic cancer |
** |
Stomach cancer |
2 |
100,701 |
significant reduction of the risk of stomach cancer |
*** |
Esophagus cancer |
2 |
100,694 |
significant reduction in the risk of cancer of the oesophagus |
* |
* : pvalue <0,05 ; ** : pvalue<0,01 ; *** : pvalue < 0.001.
Table 1. Effects of metformin on different types of cancer. (Adapted from Franciosi et al, 2013).
Mechanism of action
Therapeutical dosage recommandations
Metformin was shown to reduce visceral adiposity and insulin resistance after 8 weeks of drug therapy at dose of 850 mg, 3 times per day (Saint-Marc and Touraine, 1999).
Pharmokinetics of metformin
Bioavailability
The oral bioavailability of metformin is 55%± 16% (mean ± SD). No or low protein-binding have been reported. It is absorbed mainly by the small intestine (Graham et al, 2011).
Metabolism and Excretion
Metformin is excreted unchanged by urine. The half-life of its elimination during multiple dosages with normal renal function is about 5 hours (Graham et al, 2011).
Membrane-Transporters of Metformin
The pharmacokinetics of metformin is mainly determined by membrane transporters (Table 2).
Membrane transporter Function(s) Tissue Location(s) Plasma Membrane Monoamine Transporter (PMAT) Uptake of metformin from the gastrointestinal tract Luminal side of enterocytes.on the membrane of renal epithelial cells Organic Cation Transporters (OCTs) Modulation of the intestinal absorption, hepatic uptake, and renal excretion of metformin Membrane, brush border and cytoplasm of the enterocytes.Membrane in the renal tubules, proximal and the distal tubules in the kidney Multidrug And Toxin Extrusion (MATE) transporters Hepatic and renal excretion of metformin Liver, kidney, and skeletal muscle
Table 2. Membrane-transporters of metformin. (Adapted from Chen et al, 2013 ;Gong et al, 2013).
The pharmacologic effects of metformin are primarily exerted in the liver, at least partly via the activation of AMPK and the subsequent inhibition of gluconeogenesis (Chen et al, 2013; Gong et al, 2013).
Main actions of metformin
Microarray analyses showed that metformin induces the same gene expression profile as caloric restriction (CR), which was proved to extend life span and health span in various organisms. Metformin acts without any reduction in food intake (Novelle et al, 2016).
Most recently accepted scientific model for the global mechanim of action of metformin
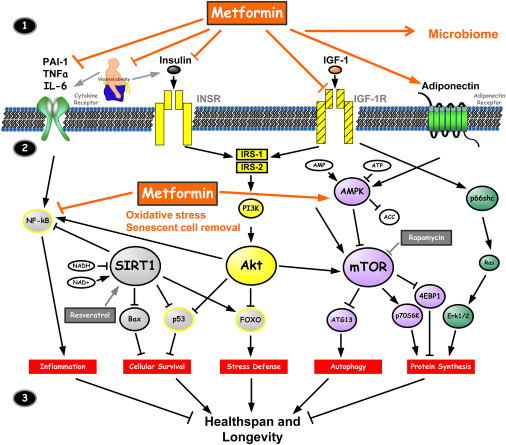
Figure 3 summarizes the cellular mechanism of action of metformin. Outside of the cell (1, top), metformin has the effects on the receptors for cytokines, insulin, IGF-1 and adiponectin. All of them may influence the longevity. Intracellularly (2, middle), metformin inhibits the inflammatory pathway and activates AMPK that inhibits mTOR. The outcome of all these processes (3, bottom) affects inflammation process, cellular survival, stress defense, cell autophagy, and protein synthesis, which have major roles associated with aging (Barzilai et al, 2012). In the following parts of the essay, the action on AMPK, insulin and cytokines will be more described. Other specific modes of action on cancer, mitochondria, vascular endothelium and female reproductive system will be mentioned.
|
Action on AMPK in the liver
Metformin treatment results in the phosphorylation and activation of AMP-activated protein kinase (AMPK) in the liver (Gong et al, 2013). AMPK acts on different receptors (Table 3). It’s a major cellular regulator of lipid and glucose metabolism (Figure 3).
Receptors of AMPK and type of modulation |
Cellular effects |
inactivates HMG-CoA reductase, MTOR, ACC-2 ACC, glycerol-3-phosphate acyltransferase, and carbohydrate response element-binding protein |
stimulation of fatty acid oxidation with inhibition of cholesterol and triglyceride synthesis. |
suppresses the expression of SREBP-1 |
suppresses key lipogenic transcription factor. |
disrupts the coactivation of PXR with SRC1 |
down-regulation of CYP3A4 gene expression. |
activates SiRT1 and increases Pgc-1a expression in the nucleus |
downstream activation of mitochondrial biogenesis. |
increasing GLUT4 translocation activity |
increase glucose uptake in skeletal muscle. |
Table 3. Receptors of AMPK and their cellular effects in the liver. (Adapted from Gong et al, 2013).
Action linked to anti-hyperglycemia effect
Metformin can reduce blood glucose level by several different mechanisms, especially through non-pancreatic mechanisms without increasing insulin secretion. It increases the effects of insulin; hence, it is termed “insulin sensitizer”. It suppresses endogenous glucose produced by the liver, due to a reduction of gluconeogenesis. It doesn't affect glycogenolysis. In addition, metformin activates the enzyme adenosine monophosphate kinase (AMPK) resulting in the inhibition of key enzymes for gluconeogenesis and glycogen synthesis in the liver while stimulating insulin signaling and glucose transport in muscles. AMPK regulates the cellular and organ metabolism and any decrease in hepatic energy will leads to the activation of AMPK (Figure 3) (Nasri and Rafieian-Kopaei, 2014).
Action linked to anti-oxidant effect
Mitochondria represents as one of the major cellular sources for ROS (Reactive Oxygen Species), with a great number of tissue pathologies, inducing oxidative stress. ROS are implicated in LPS-induced (lipopolysaccharide) IL-1β (proinflammatory cytokine) mRNA production in macrophages (Figure 3). Metformin alters ROS and cytokine production induced by TLR7/8 and TLR9 ligands, thus decreases LPS-induced ROC (Kelly et al, 2015).
Action linked to anti-cancer effect
Backdated analysis of clinical data shows us that inhibition of mTOR (mammalian Target Of Rapamycin) pathway prevents cancer in humans (Blagosklonny, 2008). It is important to know that hyperinsulinemia is also an important factor of cancer development (Anisimov, 2010). Metformin acts as an inhibitor of mTORC1 (mechanistic TOR complex 1) through both AMPK-dependent and independent mechanisms. AMPK activation inhibits the protein kinase mTOR, preventing the phosphorylation of downstream targets, including S6 kinase, ribosomal protein S6, and eIF4E-binding protein 1. Among AMPK-independent mechanisms by which metformin inhibits mTORC1 signaling, it acts to inhibit the Ras-related GTP binding (Rag) GTPases and up-regulate REDD1, a hypoxia-inducible factor 1 (HIF-1) target (Novelle et al, 2016).
Action on mitochondria complex 1
Owen, Doran and Halestrap (2000) showed that 50 mmol metformin inhibited mitochondrial oxidation of glutamate-malate metabolism in hepatoma cells by 13 and 30% after 24 and 60 hours exposure respectively. Succinate oxidation was unaffected. Metformin also leads to time-dependent inhibition of complex 1 in isolated mitochondria, whereas in submitochondrial particles inhibition requires high metformin concentrations (K(0.5),79 mM). These data are compatible with the slow membrane-potential-driven accumulation of the positively charged drug within the mitochondrial matrix leading to inhibition of complex 1 (Owen, Doran and Halestrap, 2000). Metformin inhibits mitochondrial complex I activity results in cellular respiration decreasement (Novelle et al, 2016) yielding lower ATP levels.
Action on vascular endothelium
The vascular protective actions of metformin are mediated through AMPK activation and subsequent hepatic gluconeogenesis inhibition, fatty acid oxidation as well as an insulin sensitizing action in striated muscle and adipose tissue (Triggle and Ding, 2017).
Action on female reproductive system
Metformin may improve the menstrual regularity of females with polycystic ovary syndrome leading to spontaneous ovulation, and by enhancing the induction of ovulation with clomiphene citrate (Anisimov, 2010).
Pharmacovigilance of Metformin
Side-effects of Metformin therapy
Gastro-intestinal symptoms
Acute and chronic administration of metformin cause gastro-intestinal symptoms and digestive troubles like nausea and diarrhea . It was reported such common manifestations in differents studies (Dunn and Peter, 1995 ; Jacob et al, 2018, Liu et al, 2014 ; Vecchio et al, 2014). They were estimated to occur in 20% of patients (McCreight et al, 2018; Howlett and Bayley, 1999). Liu et al (2014) reported that diarrhea appeared in 11,3 % of the metformin treated patients. Diarrhea is the most significative metformin-induced side-effects.
In most of the cases, digestive disorders are reversible. Dunn and Peter (1995) estimated a level of non-curable intolerance inferior to 5%. To reduce and eliminate them, metformin doses can be reduced (Hermann, 1979). Moreover, it is possible to avoid them if metformin is absorbed with or after food (Dunn and Peter, 1995; Hermann, 1979).The mechanism of apparition of these disorders is still debated. It may be attributed to local factors in the gut affecting the individual microbiome or intestinal receptors. Further studies are needed (McCreight et al, 2018). In addition, this common digestive incomfort could induce maladsorbtion of nutrients and vitamin deficiency.
Chronic vitamin B12 deficiency
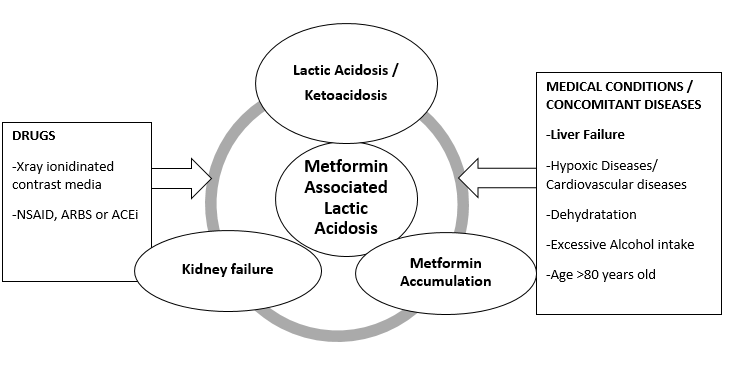
In case of long term use of metformin, cases of anemia and vitamin B12 deficiency have been described and significantly linked to the use of this drug (Hermann,1979). Metformin intake has induced a significant decrease of Vitamin B12 plasma level after 6 weeks to 208 weeks of intake ( Liu et al, 2014). Metformin Dose >2000mg/day has a greater impact on the Vitamine B12 plasma level reduction. Vitamin B12 is a vital nutrient especially for the nervous system (Liu et al, 2014) and cardiovascular system. It acts on the metabolism of a cardiovascular cytotoxic amino acid homocystein (an intermediate product of the methionine metabolism). So, metformin intake could trigger an accumulation of homocystein. Then, it could increase the risk of cardiovascular Disease (CVD). Vitamin B12 deficiency can be remedied by supplementation. Moreover, people at risk of cardiovascular disease are not recommended to use metformin (Figure 4) (Esmaeilzadeh, Gholinezhad-Chari and Ghadimi, 2017).
Hypoglycemia
As metformin has anti-hyperglycemia effects and leads to a decrease of blood sugar level, it can cause hypoglycemia or low blood sugar reaction. This condition has some unpleasant symptoms as dizziness or confusion (Jacob, Garrick and Goldberg, 2018).
Compared to the sulfonylurea (other antidiabetic drug), hypoglycemia conditions are rare and less serious with metformin (Howlett and Bayley, 1999). In that case, it generally causes a very slight hypoglycemia or not at all. It only reduces the glycemia to a normal level. This condition can be affected by several risk factors like low uptake of calories, renal and hepatic diseases, hemodialysis and some specific treatments (glimeperid) (Howlett and Bayley, 1999). Most of this conditions appeared in the contraindications of the drug (Figure 4) . So, if the recommandations are followed, it is very unlikely to cause serious problems (Howlett and Bayley, 1999).
Accumulation of acid lactic and fatal acid lactic acidosis
One of the most important and well studied potential side effects of metformin is acid lactic acidosis. Biguanide class of molecules are known to increase the plasma lactic acid level. This condition can lead to acid lactic acidosis (Vecchio et al, 2014).In most of the studies, lactic acid acidosis is considered as a rare outcome. It has an incidence of about 0.03 cases per 1000 patients-years of treatment. However, if it develops, it is fatal in the half of the cases (Howlett and Bayley, 1999). Lactic acidosis can present clinical signs like anorexia, nausea, vomiting and abdominal pain. More severe cases can be marked by hypotension, respiratory failure, arrhythmia and hypothermia (Prikis et al, 2007).
Controversial results about a link between an increase in lactic acid level in the blood and metformin uptake were found (Vecchio et al, 2014). So, a classification of the lactic acidosis types was established based on its level of association with metformin (Vecchio et al, 2014). 4 categories are distinguished from no link to a direct induction: metformin-unrelated lactic acidosis (MULA)‘metformin-induced lactic acidosis (MILA)’ (exclusively due to metformin),‘metformin-associated lactic acidosis (MALA)’ lactic acidosis in metformin therapy (LAMT) (if metformin concentration is not available). There is a complex association between acidosis and metformin admnistration (Lalau et al, 2017). A lot of factors can influence this association. They include most of the factors which are present in the contradictions of that drug (Figure 4).
|
Risk of ketoacidosis
Metformin treatment may increase blood ketoacid level (Buse et al, 2017). MALKA (Metformin Associated ketoacidosis) was reported to have fatal consequences (Schwetz et al, 2017). The ketogenesis is induced by the mechanism of action of metformin by inhibiting the hepatic gluconeogenesis (Schwetz et al, 2017; Bonsignore et al, 2014). In combination to MALA and renal diseases, other factors can influence the production of ketoacid like pregnancy and alcohol intake (Schwetz et al, 2017). So, those factors are known to be contraindications to use metformin (Figure 4).
Contraindications and Precautions for metformin treatment
Most of the contraindications listed in the prescription can cause the most detrimental side effects. So, they are in majority linked with acid lactic acidosis. Two types of contraindications can be reported : contraindications linked to the health status of the patient and contraindications linked to drug interactions (Figure 4).
Contraindications linked to the health status, medical condition of the patient
As most of metformin is eliminated by the kidney, renal disease or dysfonction is the most important risk factor that can found in the different studies as it has an impact on the clearance of the drug (Lalau et al, 2017). Renal function anomaly is generally detected when serum creatinine level > 130 micromol/L or > 1.5 g/L) . In that case, most of the metformin can't be eliminated. So, it tends to accumulate and causes the adverse effects. Hepatic disease is another key factor in the production of lactic acid (Lalau et al, 2017). As metformin increases the uptake of glucose by the liver, the liver converts extra glucose to extra lactic acid and sends it in the plasma. If the liver has impaired functions lactic acid isn't removed and is accumulated in the plasma. So, Howlett and Bayley (1999) showed that the most serious side effects occur if the patients are wrongly prescribed the drug. It only happens if the recommandations and contraindications are not respected. Other contradictions like congestive heart failure which are in link with hypoxic states have to mentioned. Some characteristics of the patient (age and pregnancy) should be checked as well. This conditions have an influence on the normal function of the kidney and may influence the acid lactic accumulation (Figure 4) (Lalau et al, 2017).
Contraindications due to Drug to Drug interactions
One of the most well-known drug interactions to metformin is the use of ionidated contrast media. That kind of treatment increases the risk of lactic acidosis (Figure 4). Medications that deteriorate renal functions like angiotensin-converting enzyme inhibitors ACEi, angiotensin receptor blockers, ARB and non-steroidal anti-inflammatory drugs NSAI have to be avoided (Figure 4) (Visconti et al, 2016). Other drug like carbose ; cephalexine cimetidine ; dolutegravir ; pyramethamine, ranolazine; trimethoprim and tyrosine kinase inhibitors treatment are included in the exclusion factors of some studies (McCreight et al, 2018). They are known to influence the pharmacokinetics of metformin. Drug to Drug interactions between metformin and other drugs are still discussed. Individual factors and pharmacogenetics of metformin require further studies (Visconti et al, 2016) .
Conclusion
In most of studies, there is a significant association between metformin therapy and anti-aging effects. It reduces or delays the age-related diseases like most of the fatal cancers. So, it may significantly increase the lifespan and the living conditions of older people. Concerning the laboratory studies on animals, results are controversial. Individual factors, age, sex, species and strains, genetic factors, mode of consumption of metformin (duration, dosage, age when to start the therapy) may have a significant impact on the results . Only, studies on female mice of specific strains significantly showed an increase in lifespan and a reduction in age-related cancers. Metformin is an oral drug which is not metabolized and is excreted unchanged in the urine. Its mechanism of action is not totally understood. Metformin mainly acts on the body by stimulating a membrane or intracellular receptor called AMPK . Then, AMPK activates or inhibits other receptors and pathways in different parts of the body. Their final effects is associated to cellular wellbeing and longevity. Metformin therapy may cause several and a great variety of positive effects and advantages. Nevertheless, some adverse effects were reported. Gastro-intestinal problems are the most frequent disorders but they are reversible and not too severe. Individual risk factors like principally hepatic or renal diseases can influence the occurence of side effects. Metformin intake was proved to be in relation or to cause fatal consequences. Acid Lactic acidosis is the most significant fatal outcome. Nevertheless, if metformin is taken while respecting the recommendation of use, it is very unlikely to cause any serious and irreversible problems. Other individual pharmacogenetic factors require further studies to explain the heterogenous inter-individual answer to metformin. Compared to other anti-hyperglycemic drug like other common biguanides (phenformin), the side effects are relatively minimal. So, the benefit/risk ratio is significantly superior. Metformin can be considered as a safe drug (Hermann, 1979). New studies suggest that metformin could have a positive effect on other systems and tissues like the skin, cognitive functions or even the mood. Thus, all the secrets of metformin and fields of application are far from being completely known and understood.
References
Anisimov, V. N. (2013): Metformin: Do we finally have an anti-aging drug? Cell Cycle 12: (22) 3483–3489
Anisimov, V. N. (2010): Metformin for aging and cancer prevention. Aging (Albany NY) 2: (11) 760–774
Algire, C.; Moiseeva, O.; Deschênes-Simard, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Viollet, B.; Ferbeyre G.; Pollak M. N. (2012): Metformin Reduces Endogenous Reactive Oxygen Species and Associated DNA Damage.Cancer Prevention Research 5: (4) 536-543
Barzilai, N.; Crandall, J. P.; Kritchevsky, S. B.; Espeland, M. A. (2016): Metformin as a Tool to Target Aging. Cell Metabolism 23: (6) 1060-1065
Blagosklonny M. V. (2008): Prevention of cancer by inhibiting aging. Cancer Biology Therapy 7: (10) 1520-4
Bonsignore, A.; Pozzi, F.; Fraternali Orcioni, G.; Ventura, F.; Palmiere, C. (2014): Fatal metformin overdose: case report and postmortem biochemistry contribution. International Journal of Legal Medicine 128: (3) 483–492
Buse, J.B.; DeFronzo, R.A.; Rosenstock, J.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. (2016): The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care 39: 198
Chen, S.; Zhou, J.; Xi, M., Jia, Y.; Wong, Y.; Zhao, J.; Ding, L.; Zhang, J.; Wen, A. (2013):Pharmacogenetic variation and metformin response. Current Drug Metabolism 14: (10) 1070-82
Dunn, C.J.; Peters, D.H.(1995): Metformin. A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs 49: (5) 721-49
Esmaeilzadeh, S.; Gholinezhad-Chari, M.; Ghadimi, R. (2017): The effect of metformin treatment on the serum levels of homocysteine, folic acid, and vitamin B12 in patients with polycystic ovary syndrome. Journal of Human Reproduction Sciences 10: 95-101
Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G. F. M.; Pellegrini, F.; Nicolucci, A. (2013): Metformin Therapy and Risk of Cancer in Patients with Type 2 Diabetes: Systematic Review. Plos One: (8) e71583
Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. (2012): Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics 22: (11) 820-7
Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; Ray, J.E.; Timmins, P.; Williams, K.M. (2011): Clinical Pharmacokinetics of Metformin. Clinical Pharmacokinetics 50: (2) 81–98
Hermann, L.S. (1979): Metformin: a review of its pharmacological properties and therapeutic use. Diabete Metabolism 5: (3) 233-45
Howlett, H. C.; Bailey, C.J. (1999): A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug Safety 20: (6) 489-503
Jacob, T.; Garrick, R.; Goldberg, M.D. (2018): Recurrent lactic acidosis and hypoglycemia with inadvertent metformin use: a case of look-alike pills. Endocrinology, Diabetes & Metabolism Case Reports: 17-0148
Kelly, B.; Tannahill, G. M.; Murphy, M. P.; O'neill, L. A. (2015): Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1β (IL-1β) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-activated Macrophages. Journal of Biology Chemistry 290: (33) 20348-59
Lalau, J. D.; Kajbaf, F.; Protti, A.; Christensen, M.M.; De Broe, M. E.; Wiernsperger, N. (2017): Metformin‐associated lactic acidosis (MALA): Moving towards a new paradigm. Diabetes, Obesity and Metabolism 19: 1502–1512
Liu, C.; Wu, D.; Zheng, X.; Li, P.;. Li, L. (2014): Efficacy and Safety of Metformin for Patients with Type 1 Diabetes Mellitus: A Meta-Analysis. Diabetes Technology & Therapeutics 17: 142–148
Martin-Montalvo, A. ; Mercken, E. M. ; Mitchell, S. J. ; Palacios, H. H. ; Mote, P. L. ; Scheibye-Knudsen, M. ; de Cabo, R. (2013): Metformin improves healthspan and lifespan in mice. Nature Communications 4: 2192
McCreight, L. J.; Stage, T. B.; Connelly, P.; Lonergan, M.; Nielsen, F.; Prehn, C.; Adamski, J.; Brøsen, K.; Pearson, E. R. (2018): Pharmacokinetics of metformin in patients with gastrointestinal intolerance. Diabetes, Obesity and Metabolism : 1-9
Nasri, H.; Rafieian-Kopaei, M. (2014): Metformin: Current knowledge. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences 19: (7) 658–664
Novelle, M. G.; Ali, A.; Diéguez, C.; Bernier, M.; De cabo, R. (2016): Metformin: A Hopeful Promise in Aging Research. Cold Spring Harbour Perspectives in Medicine 6: (3) a025932
Owen, M. R.; Doran, E.; Halestrap, A. P. (2000): Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochemistry Journal 348: (3) 607-14
Patil, S. P.; Jain, P. D.; Ghumatkar, P. J.; Tambe, R.; Sathaye, S. (2014): Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277: 747–754
Prikis, M.; Mesler, E. L.; Hood, V. L.; Weise, W. J.; (2007): When a friend can become an enemy! Recognition and management of metformin-associated lactic acidosis. Kidney International 72: 1157–1160
Pryor, R.; Cabreiro, F. (2015): Repurposing metformin: an old drug with new tricks in its binding pockets. Biochemical Journal 471: (3) 307–322
Saint-Marc, T.; Touraine, J.L. (1999): Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS 13: (8) 1000-2
Schwetz, V.; Eisner, F.; Schilcher, G.; Eller, K.; Plank, J.; Lind, A.; Pieber, T. R.; Mader, J. K.; Eller, P. (2017): Combined metformin-associated lactic acidosis and euglycemic ketoacidosis. Wiener Klinische Wochenschrift 129: 646–649
Stage, T. B.; Brøsen, K.; Christensen, M. M. H.(2015): A Comprehensive Review of Drug–Drug Interactions with Metformin. Clinical Pharmacokinetics 54: 811–824
Triggle, C. R.; Ding, H. (2017): Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiologica (Oxford) 219: (1) 138-151
Vecchio, S.; Giampreti, A.; Petrolini, V. M.; Lonati, D.; Protti, A. ; Papa, P. ; Rognoni, C.; Valli, A.; Rocchi, L.; Rolandi, L.; Manzo, L.; Locatelli, C. A. (2014): Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clinical Toxicology 52: (2) 129-35
Visconti, L.; Cernaro, V.; Ferrara, D.; Costantino, G.; Aloisi, C.; Amico, L.; Chirico, V.; Santoro, D.; Noto, A.; David, A.; Buemi, M.; Lacquaniti, A. (2016): Metformin-related lactic acidosis: is it a myth or an underestimated reality? Renal Failure 38: 1560–1565
Wang, S. B.; Lei, K. J.; Liu, J. P.; Jia, Y. M. (2017): Continuous use of metformin can improve survival in type 2 diabetic patients with ovarian cancer: A retrospective study. Medicine 96: (29) e7605