The Role of Gut Microbiome in Anxiety Disorders and Depression
Authors: Robin Luttekes, Nora Mørk, Hannah Nummedal
Supervisor: Dr. Zoltán Balázs Bárány
The gut microbiome describes the symbiotic microorganisms that inhabit the gastrointestinal (GI) tract, and are acquired at birth (Flux and Lowry, 2020; Li et al, 2018; Lach et al, 2018). The gut microbiota plays a large role in human health, aiding in maintaining intestinal homeostasis, protection against pathogens, and helping with the digestion of food, which in turn can influence the physiology of the host cells, including the brain (Peirce and Alviña, 2019; Tetel et al, 2017). In recent years, more evidence shows the involvement of the gut microbiota in the gut-brain axis, which is the bidirectional communication between the enteric nervous system (ENS) and the central nervous system (CNS). Microbe peptides can regulate gut peptide hormones, modulating the signalling to the brain directly or indirectly (Lach et al, 2018).
Changes in the gut microbiome composition can lead to psychiatric behaviours such as anxiety and depression via the gut-brain axis (Lach et al., 2018). Stress-related effects and inflammation in the GI tract are two large factors that can influence the microbiome composition and the communicating pathways, and often causes depression and anxiety (Foster et al, 2017).
Contents
Gut Microbiota
The terms “microbiome” and “microbiota” are often interchangeable, however, in this paper “microbiome” will refer to the collective population of symbiotic microbes whereas “microbiota” will refer to the organisms themselves (Flux and Lowry, 2020).
The gastrointestinal (GI) microbiome in mammals begins at birth via the exposure of the mother’s microbiota in the placenta, umbilical cord blood, amniotic fluid and foetal membranes. The mode of delivery and other factors, such as breastfeeding, can influence the gut microbiome diversity (Lach et al., 2018). For example, with vaginal birthing, infants will have a high concentration of Lactobacilli during the early developing stage due to there being an abundance of Lactobacilli in the vaginal canal. Within 2.5 years’ time, the composition of the gut microbiome will represent that of an adult (Cossuto et al., 2018). In healthy human adults, there are 2 dominating phyla which makes up more than 70% of the gut microflora. These are the Firmicutes and Bacteroides (Peirce and Alviña, 2019).
There are many factors, such as stress and diet, that can cause changes in the composition of the gut microbiome. Shifts in the gut microbiome can cause physiological modifications to the brain via the different pathways, causing anxiety and depression (Flux and Lowry, 2020).
Communicating Pathways of Microbiota-Gut-Brain Axis
The complex communication network that makes up the microbiome-gut-brain (MGB) axis is composed of 3 bi-directional pathways. These include the enteric nervous system, the neuroendocrine signaling pathways, and the neuroimmunology systems (Foster et al, 2017; Li et al, 2018). Through these systems, microbiota can exert their effects and alter GI and brain functions (Foster et al, 2017). Due to the bi-directional characteristic, the CNS can also regulate the microbiome composition (Li et al, 2018).
Enteric nervous system
The gut is innervated by the enteric nervous system (ENS), a division of the autonomic nervous system (ANS) and is made up of parasympathetic and sympathetic fibres. Whilst the ENS can regulate basic GI functions, such as mucous secretion and blood flow, the central control of gut functions is supplied by the vagus nerve, which also plays an important role in gut adaptive responses during periods of stress (Foster et al, 2017). The sensory neurons of the ENS are exposed to the gut microbiota. These neurons are in contact with motor neurons that are involved in the regulation of intestinal motility and gut hormone secretion. These neurons also form synapses with the vagal nerve (Li et al, 2018). The vagal nerve connects the gut to the brain, serving as an important neural pathway for the MGB communication (Foster et al, 2017; Li et al, 2018).
Microbiota effects on the ENS can include increase in excitability and frequency of action potential by targeting ion channels in enteric sensory neurons, as demonstrated by probiotic Lactobacillus reuteri in mice (Foster et al, 2017).
Neuroendocrine signaling pathway
Peptides of the gut play an important role in the MGB axis via the neuroendocrine signaling pathway (Foster et al, 2017). Specialised enteroendocrine cells (EECs) make up 1% of the gut epithelium and are responsible for the gut endocrine system (Lach et al, 2018). There are over 20 types of EECs in humans, each producing different peptides (Li et al, 2018). In total, there are over 100 signaling peptides that play a role in the gut-brain axis (Lach et al, 2018).
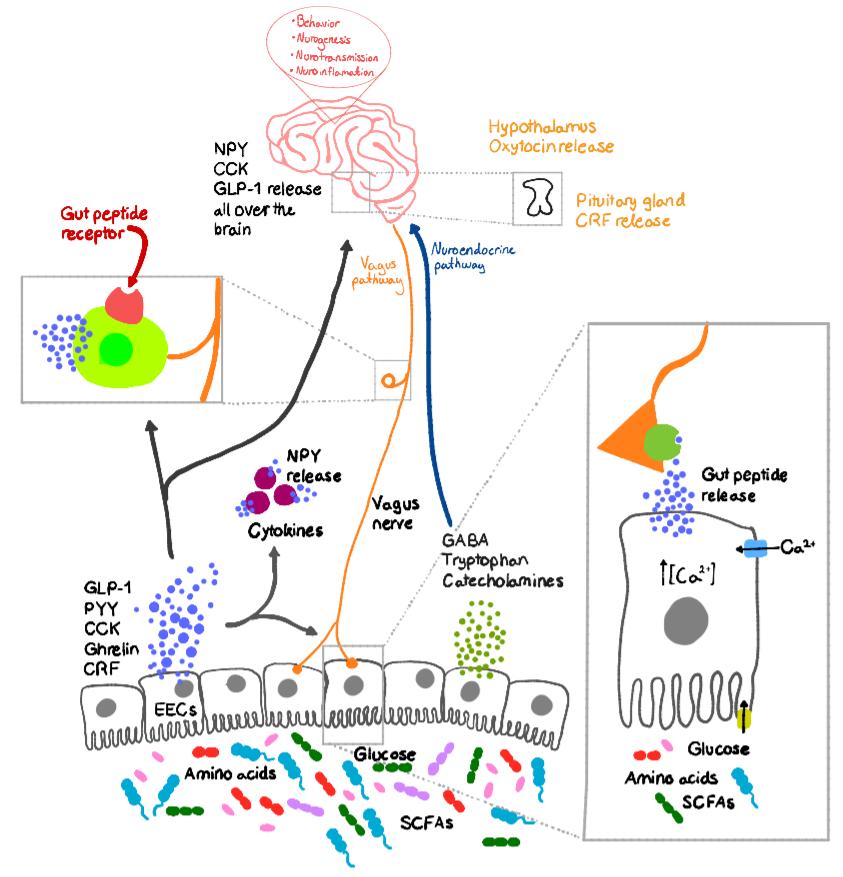
Bacteria by-products can influence the production of neuropeptides, such as peptide YY (PYY), neuropeptide Y (NPY), cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), corticotropin-releasing factor (CRF), γ-amino butyric acid (GABA), and substance P, when coming into contact with the EECs. This mechanism is demonstrated in Figure 1 regarding as to how the neuropeptides released can influence the physiology of the brain via different pathways directly or indirectly (Lach et al, 2018). After secretion, the gut peptides will diffuse through the lamina propria, which is filled with immune cells, and from there, enter the bloodstream. In the bloodstream, the peptides can either exert their effects on intrinsic ENS neurons or extrinsic neural innervation, e.g. vagal nerve, via receptors (Foster et al, 2017; Lach et al, 2018). Some gut peptides can have a direct effect by diffusion through the selective semi-permeable blood brain barrier (BBB) via specific transport channels (Lach et al, 2018).
|
Figure 1. Neuropeptides affecting brain via ANS and neuroimmunology pathway |
SCFA = short-chain fatty acid |
(adapted from Lach et al, 2018) |
Neuroimmunology pathway
The immune system plays an important role in the MGB axis, interacting with the hypothalamic-pituitary-adrenal (HPA) axis, ANS and ENS directly. The gut itself plays an important role as a defensive barrier to externally acquired pathogens and with the gut-associated lymphoid tissues forming the largest immune organ, making up more than 70% of the immune system (Foster et al, 2017). Microbiota influence immune cells’ production of cytokines, which may be pro-inflammatory or anti-inflammatory, and may cause physiological effects such as depression (Foster et al, 2017; Lach et al, 2018; Li et al, 2018).
Cytokines are important mediator molecules in the bi-directional communication pathway between the immune system and the brain. They are inflammatory molecules and are released both centrally and peripherally (Flux and Lowry, 2020).Cytokines can either exert their effects via the vagus nerve or cross through the BBB by means of distinct active transport molecules located along the barrier (Flux and Lowry, 2020; Ünsalver and Ceylan, 2020).
Depression and Anxiety
Approximately 4.4% of the world's population suffer from depression (Flux and Lowry, 2020). Depression typically includes symptoms such as self-fulfilling negative expectation and is tied to insomnia (Kendler et al, 2010; Li et al, 2018). It is a state caused by many factors at a biochemical level (Kendler et al, 2010). Symptoms of generalized anxiety disorder (GAD) include the feeling of persistent restlessness, fatigue, and a difficulty focusing (Tyrer and Baldwin, 2006). Anxiety and depression are both defined as a stress-related disorder. However, although GAD and depression are typically triggered by certain situations, they are not connected to stress per se as stress, alone, is not enough to cause these disorders (Nester et al, 2002; Tyler and Baldwin, 2006).
Aspects of depression and anxiety
In most studies of depression today, the main focus is the brain-based understanding of depression. One of the more recognized theories on depression is the theory of “Chemical imbalance”. This theory states that depression is related to the imbalance of particular neurotransmitters. However, this theory does not effectively explain the etiology/cause of depression and development of treatments. The understanding of Major depressive disorder (MDD) is currently not only alterations in neurotransmitters, but also changes in neural circuits, and shifts in the function of the immune system and changes in the endocrine system (Flux and Lowry, 2020).
Major depressive disorder can cause structural alterations in the brain, which can be viewed with different imaging techniques. Changes in the brain of patients with MDD are: decreased in volume in the prefrontal cortex, basal ganglia, anterior cingulate cortex, hippocampus and thalamus (Flux and Lowry, 2020).
Gut microbiome influence on depression and anxiety
There is increasing evidence from different studies that suggests a link between the gut microbiome composition and the development of psychiatric disorders, including depression and anxiety (Pierce and Alviña, 2019). There are several animal and a few human studies that demonstrate this connection between the gut microbiome and anxiety and depressive-like behaviours (Flux and Lowry, 2020). Depression and GAD are often connected with gastrointestinal disturbances (Li et al, 2018). These GI disturbances include irritable bowel syndrome (IBS), inflammatory bowel disease (IDB), which can be influenced by the gut microbiota (Lach et al, 2018).
A study on the microbiota in healthy and depressed patients showed that participants with IBS and participants with major depressive disorder (MDD) and participants with both have similar microbiota compositions. In patients suffering from MDD, the most typical gut microbiome alteration is characterized by inferior bacterial diversity and an increased representation of the bacterial genera Bacteroides or Prevotella. These bacteria are responsible for converting tryptophan, which is the only precursor for production of serotonin, to indole. This will affect the availability of tryptophan, which can lead to the serotonergic imbalance observed in patients with MDD (Inserra et al, 2018). Majority of serotonin (5-HT) is produced by the ECCs and in the gut, it helps with the regulation of GI secretion, whereas in the brain, it plays a role in regulating mood and cognition. The lack of serotonin caused by Bacteroides and Prevotella may contribute to mood disorders (Foster et al, 2017; Inserra et al, 2018).
The bacteria Oscillibacter was also observed to be increased in MDD patients, which are responsible for producing valeric acid, a short fatty chain acid that resembles the inhibitory neurotransmitter GABA and is further involved in gastrointestinal functions. The genus Faecalibacterium was reduced in the participants with MDD, which is known for its anti-inflammatory properties. This coincides with the increased inflammation in the colon (Inserra et al. 2018).
Stress and Inflammation Leading to Depression and Anxiety
There have been many animal and human studies that demonstrate physiological pathways involving inflammatory and stress responses play a role in the causation of depression and anxiety (Pierce and Alviña, 2019). The effects of stress-induced inflammation affect the brain via the neuroimmune pathway.
Stress-induced inflammation in depression and anxiety
Stress can activate the immune system through the hypothalamic-pituitary-adrenal (HPA) axis, which through a cascade mechanism of hormones prepare the body for an adequate response to the stress stimuli. The hypothalamus is signalized to release corticotropin-releasing hormone (CRH), which will further signal the pituitary gland to release adrenocorticotropic hormone (ACTH). In the circulation, ACTH will signal for the adrenal cortex to release the stress factor hormone cortisol in humans (Flux and Lowry, 2020).
The hyperactivity of the HPA axis has been repeatedly reported in depressed patients, especially in the melancholic subtype (Farzi et al, 2018). In early studies of the HPA axis, dexamethasone suppression test was used to study the correlation between the HPA axis and depression. Dexamethasone is a synthetic glucocorticoid and will normally induce a negative feedback in the HPA axis, reducing the cortisol secretion the next day. It was found that in patients with depression, the HPA responded less to the negative feedback, resulting in a smaller decrease of cortisol secretion after introduction of dexamethasone (Flux and Lowry, 2020).
Stress or chronic stress in young age groups are risk factors for developing depression and irritable bowel syndrome (IBS) (Farzi et al, 2018). IBS is a functional gastrointestinal disorder associated with visceral pain and changes in bowel movement, and always co-occurs with anxiety and depression. In animal studies, it is shown that gut microbiota are involved with the visceral hypersensitivity (Foster et al, 2017). In these studies, faecal microbiota from patients diagnosed with IBS were transferred to germ free (GF) mice with or without anxiety behaviour. The GF mice showed intestinal barrier dysfunction, innate immune activation and anxiety-like behaviours (Farzi et al, 2018; Foster et al, 2017).
Stress impacts colonic motor activity, changing the composition of the gut microbiome, specifically lowering the number of Lactobacillus. Patients with IBS have comparatively a lower amount of Lactobacillus and Bifidobacterium compared to healthy patients, as well as an increased ratio of Firmicutes to Bacteroidetes. In another study, patients who suffer from anxiety and depression showed improvement with consumption of Lactobacillus and Bifidobacteria (Foster et al, 2017).
Under stress, the endocrine pathways will alter the immune response, both centrally and peripherally. This will cause a series of processes that lead to neuroinflammation. The result of neuroinflammation is depression, stress or anxiety behaviours (Rea et al, 2016).
Inflammation pathways
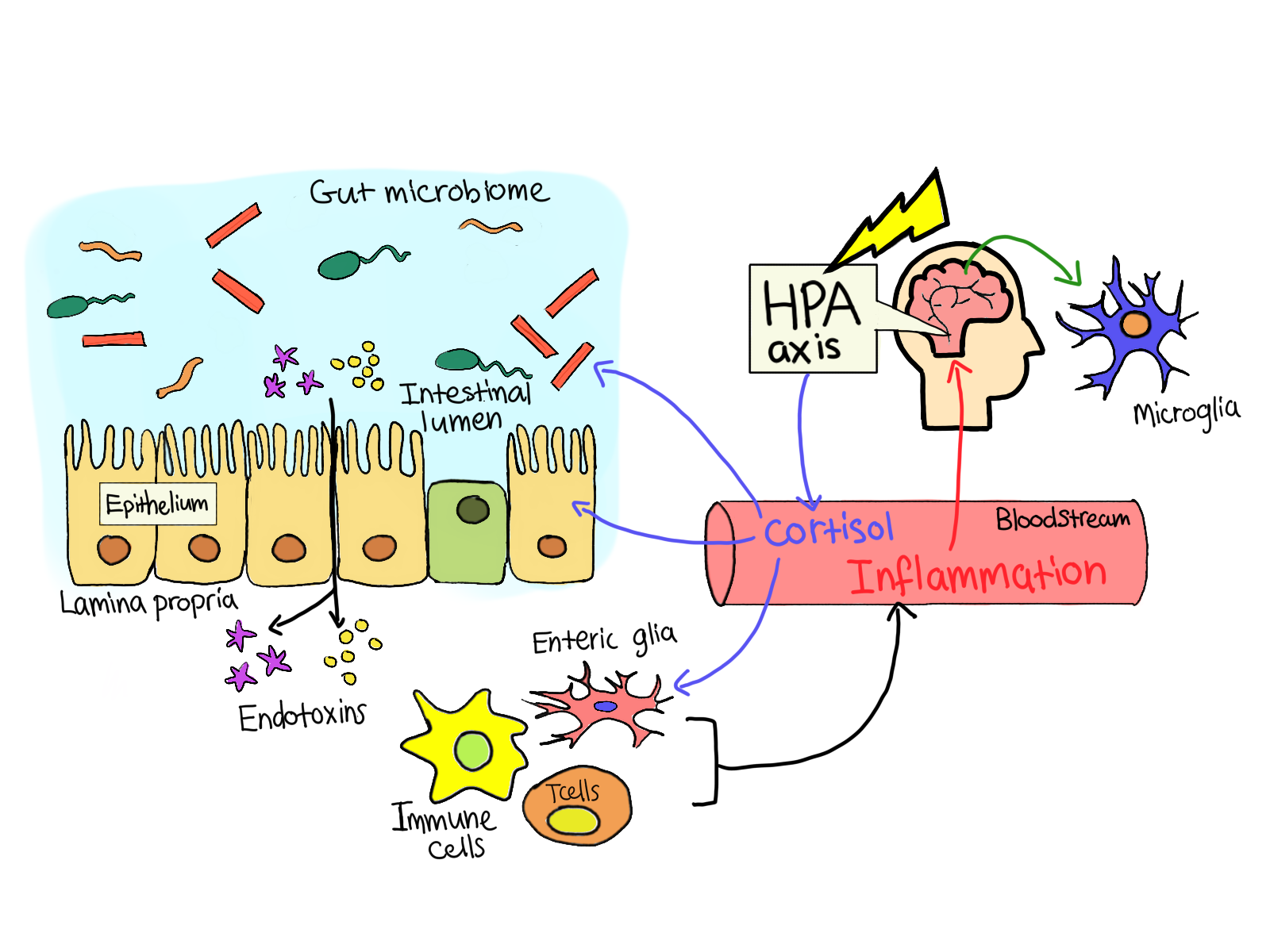
|
Figure 2. Microbiota inducing inflmattion via immune cells, causing cortisol production via HPA axis |
(adapted from Pierce and Alviña, 2019) |
Inflammation is a result of the immune system producing cytokines in response to a stresser (Flux and Lowry, 2020). The imbalance in gut microbiota, where there is an increase in pro-inflammatory bacteria, causes increased permeability of the gut barrier, a phenomenon called “leaky gut” (Li et al, 2018). This disruption in the gut barrier allows for gram-negative bacteria to cross the mucosal lining and come into contact with immune cells and the ENS as seen in Figure 2 (Forster et al, 2017). The translocation of bacteria due to leaky gut phenomenon can induce immune cells to produce cytokines, which are inflammatory molecules. These molecules are released both centrally and peripherally and affect the brain through the stimulation of the vagus nerve and crossing over the BBB via transport molecules. Cytokines can cause depression, lethargy, decreased appetite, heightened sensitivity to pain, difficulty concentrating and feeling of discomfort, which are all symptoms of MDD. Some important immune-modulating cytokines include interleukin(IL)-1β, IL-6, and tumor necrosis factor (TNF) (Flux and Lowry, 2020).
Stress factors can induce a shift in the gut microbiome resulting in the upregulation of proinflammatory pathways. These pathways are mediated by Nod-like receptors such as NLRP3, an intracellular immune sensor inflammasome, detecting stress, anger or damage. Stress factors can increase the NLRP3 signaling, which will activate the IL-1β and tumor necrosis factor driven pathways, and can alter brain functions.
IL-1β is a crucial pro-inflammatory cytokine responsible for host-responses to infection and injury and crucial for the gut-immune-brain axis communication (Inserra et al. 2018; Lopez-Castejon and Brough, 2011). Exposure to stress will activate the NLPR3 signaling, which will activate the IL-1β and tumor necrosis factor-mediated pathways. The increase in the proinflammatory signaling will activate the HPA axis, aggravating depression and anxiety-like behaviours. However, how intense the NLPR3 signaling is activated is strongly influenced by the microbiota composition (Inserra et al. 2018).
Clinically diagnosed anxious patients have a higher amount of pro-inflammatory cytokines in comparison to healthy individuals. In studies involving rats, stress-induced IL-6 activity causes an alteration in gene expression in monocytes leading to anxiety-like behaviours (Pierce and Alviña, 2019).
Based on the relationship between the immune system and depression, many patients diagnosed with major depressive disorder are treated with anti-inflammatory properties. Some types of antidepressants will reduce the production of endogenous proinflammatory cytokines, and can further even alter the immune reactivity in the central nervous system (Flux and Lowry, 2020). Modern treatments can introduce anti-inflammatory properties in the treatment against depression, such as cytokine inhibitors, non-steroidal anti-inflammatory drugs, and even anti-epileptics. However, as some patients have shown no or even aggravated symptoms after anti-inflammatory treatment, the treatment should be used with caution (Flux and Lowry, 2020).
Conclusion
There appears to be a strong link between gut microbiota and psychiatric disorders such as depression and anxiety. The correlation can be seen in the gut microbiota composition between healthy individuals and patients who suffer from depression, anxiety, or IBS. This is also evident in animal models, such as GF mice.
The bi-directional communication of the microbiota-gut-brain axis is complex and involves the ENS, neuroendocrine and the neuroimmunology pathways. There are many overlapping components between these pathways, triggering a cascade of events that lead to the change of brain physiology. Many factors can trigger this, especially stress which causes the activation of the immune system via exposure of gut microbiota, leading to stress-induced inflammation. Cytokines trigger the HPA axis directly through BBB or indirectly via activation of the vagus nerve. The hyperactivity of the HPA axis has been a recurrent observation in depressed patients.
With new research and a better understanding of these mechanisms, new treatments for depression and anxiety-related disorders can be uncovered by the usage of drugs with anti-inflammatory properties. However, the complexity of the immune system is still not fully understood, and these treatments are preceded with caution. It should also be noted that there is a lack of research regarding the impact of gut microbiota on depression and anxiety in humans, especially regarding stress exposure.
References
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J. K.; Kaye, A. D. (2020): Current Perspectives on Gut Microbiome Dysbiosis and Depression. Advances in Therapy, 1-19.
- Cusotto S.; Clarke, G.; Dinan T.G.; Cryan J.F. (2018): Psychotropics and the Microbiome: a Chamber of Secrets. Psychopharmacology 236:1411-1432
Evrensel, A.; Ünsalver, B. Ö.; & Ceylan, M. E. (2019): Neuroinflammation, gut-brain axis and depression. Psychiatry investigation 17:(1) 2-8
Farzi, A.; Fröhlich, E. E.; & Holzer, P. (2018). Gut microbiota and the neuroendocrine system. Neurotherapeutics, 15:(1) 5-22.
- Flux, M. C.; Lowry, C. A. (2020): Finding intestinal fortitude: Integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiology of disease 135, 104578.
Foster J.A.; Rinaman L.; Cryan J.F. (2017): Stress & the Gut-Brain Axis: Regulation by the microbiome. Neurobiology of Stress 7:124-136
- Inserra, A.; Rogers, G. B.; Licinio, J.; Wong, M. L. (2018): The microbiota‐inflammasome hypothesis of major depression. Bioessays 40:(9) 1800027.
- Johnson, K. V. A. (2020): Gut microbiome composition and diversity are related to human personality traits. Human Microbiome Journal 15: 100069.
- Kendler, K. S.; Zachar, P.; Craver, C. (2011): What kinds of things are psychiatric disorders?. Psychological medicine, 41:(6) 1143-1150.
- Lach G.; Schellekens H.; Dinan T.C.; Cryan J.F. (2018): Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotheraputics 15:36-59
- Li, Y.; Hao, Y.; Zhang, B. (2018): The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 9:669
Nestler, E. J.; Barrot, M.; DiLeone, R. J.; Eisch, A. J.; Gold, S. J.; Monteggia, L. M. (2002): Neurobiology of depression. Neuron, 34:(1) 13-25.
- Peirce J.M.; Alviña K., 2019: The Role of Inflammation and the Gut Microbiome in Depression and Anxiety. J Neurosci Res 97:(10) 1223-1241
- Rea, K.; Dinan, T. G.; Cryan, J. F. (2016): The microbiome: a key regulator of stress and neuroinflammation. Neurobiology of stress 4: 23-33.
- Tyrer, P.; Baldwin, D. (2006): Generalised anxiety disorder. The Lancet, 368:(9553) 2156-2166.