Interaction between the gut microbial and the mucosal immune system
Elizabeth Ryan , Sunniva Gjøen Rydjord & Cathal Walsh
Contents
-
Interaction between the gut microbial and the mucosal immune system
- Introduction
- Commensal vs pathogenic bacteria
- Effector and induction site
- Defensin
- Effects of a compromised immune system on the gut microbiome
- Factors affecting homeostasis of microbiota
- Methods of the gut microbiota modulation
- Diseases caused by gut microbiome dysbiosis
- Conclusion
- References
- Figures
Introduction
The largest symbiotic ecosystem can be found in the gastrointestinal tract extending from the oral cavity to the anus. The main physiological function is the digestion of food, absorption of nutrients and elimination of waste products. The gastrointestinal tract is the entrance of viral and bacterial pathogens and imbalance in this microbiome can lead to disruption of the intestinal homeostasis. This is caused by the disproportion of the commensal and the pathogenic microbes (Li et al., 2017). The gut microbiome consists of virus, bacteria and fungi with a wide range of bacteria up to 1,500 species, however, research have shown that the individual composition of the microbial environment is affected by different environmental factors and consist of around 160 species in each individual. As a part of the mucosa-associated lymphoid tissue (MALT) the gut-associated lymphoid tissue (GALT) is an important factor in the regulation and acts as a protective barrier for the gastrointestinal tract. Important components for the protection of the gut are mucosal immune cells, immune organs, cell receptors, cytokines and Payer’s patches among with the lamina propria and epithelia. (Tokuhara et al., 2019).
Commensal vs pathogenic bacteria
Commensal bacteria are virus, fungi and bacteria that inhabit the host without causing harm, and they are part of the normal microflora with a close relationship to the host animal. They consist of microorganisms present on epithelial-covering surfaces like the gastrointestinal tract, respiratory tract, vagina etc. The microbiota co-evolved with the host and contains components able to activate adaptive and innate immunity. However, unlimited immune activations from commensal bacteria can pose risk of inflammation. Consequently, this requires a well-regulated control mechanism for tolerating non-dangerous antigens, food, and commensal bacteria which is done by the mucosal immune system. On the other hand, the mucosal immune system must exhibit a local defense-mechanism to threats like invading pathogens and this is fulfilled by the developed innate immune system which ensures proper function of the mucosal barrier. (Tlaskalová-Hogenová et al., 2004). Pattern-recognizing receptors (PRRs) have a major role in the adaptive and innate immunity. In the group covered by PRRs, toll-like receptors (TLRs) can be found. They are expressed immune cells and some non-immune cells like the intestinal epithelium. TLRs can distinguish different pathogens based on the molecular signatures, the so-called pathogen-associated molecular pattern (PAMP). PRR signalling result in the production of different proinflammatory cytokines and interferons (Bertics et al., 2013). In the symbiosis between the microbiota and the host the TLR-based recognition is important for the regulation of immune response in the intestine.
In the intestinal lumen, the intestinal epithelial cells are the first line of defence against pathogens. The intestinal epithelial cells are composed of different cells like enterocytes, Paneth cells, plasma cells and goblet cells. These cells cooperate to maintain intestinal homeostasis and promote host defence. The cells stimulated by pathogens and other antigens can be termed immunoreactive, and the intestinal mucosal immune response is induced by the local mucosal tissues where the immunoreactive cells are found. Plasma cells located in the intestinal lamina propria produce secretory immunoglobulin A, which is the main effector of mucosal response. Secretory immunoglobulin A (sIgA) neutralize bacterial toxins in the lumen and protects the mucosal surface and by this also function to maintain the immune stability and resisting pathogens. (Peng et al., 2021).
Effector and induction site
The function of the intestinal mucosal immune system can be divided into two, the induction and effector sites by function and tissue structure. GALT is mainly the induction site, which is composed of mesenteric lymph nodes and Peyer’s patches. Peyer’s patches have microfold cells which function to shuttle antigens into the Peyer’s patches for appropriate immune response. In addition, Payer’s patches generate Ig-A producing plasma cells for T-cell responses in the gut. The induction sites also have antigen presenting cells including dendritic cells, intestinal epithelial cells and macrophages. The effector sites are found in the lamina propria and in the epithelial layer with lymphocyte cells. Antigens are presented by antigen-presenting cells to the effector cells, which result in the production of antibodies and an immune response. (Peng et al., 2021).
The sampling of commensal bacteria is a mechanism where the commensal bacteria provide colonization resistance to antigens. Special receptors allow mucosal dendritic cells to extend into the intestinal lumen to engulf bacteria. From the intestine the bacteria are transported to the mesenteric lymph nodes where they selectively induce production of IgA by plasma cells. The antibodies bind and modulate the composition of the gut microbiota which limits inflammatory response and prevents penetration of pathogenic and commensal bacteria. This sampling of commensal bacteria also induces differentiation of T-cells subsets (Peng et al., 2021).
Defensin
Enteric defensin is another effector molecule with an important role of shaping the microbiota and inhibiting pathogen growth. Defensin are produced by Paneth cells located in the intestinal epithelium and functions to inactive bacteria by damaging the cell wall. Studies have shown that the composition of gut microbiota can drastically change in mice engineered to express human alpha-defensins. These examples show how important the relationship between the host and the commensal bacteria are to keep the gut mucosal environment stable (Tokuhara et al., 2019; Peng et al., 2021).
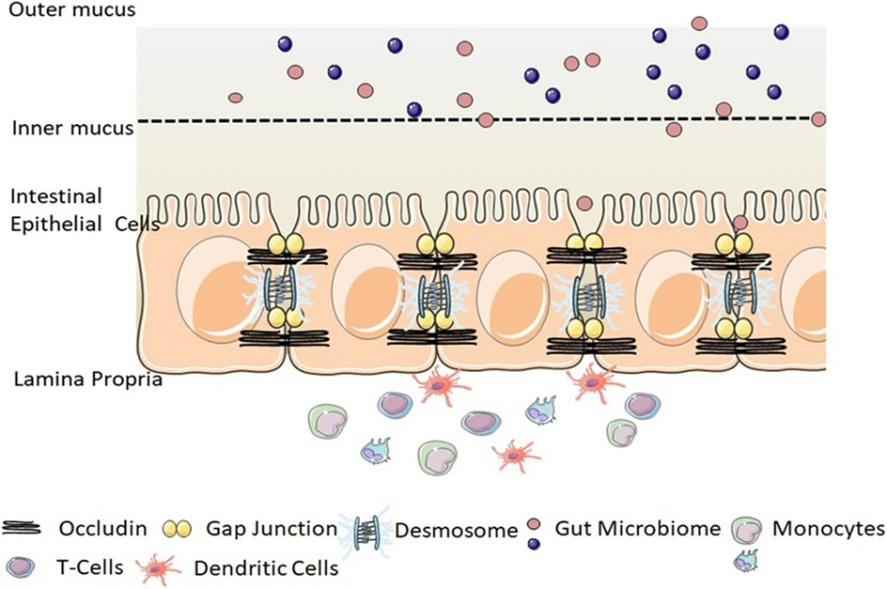
Figure 1. Intestinal epithelial barrier (Chelakkot et al 2018)
Effects of a compromised immune system on the gut microbiome
The intestinal mucosal immune system is the largest immune component in vertebrates and has a very important relationship with intestinal microflora. Intestinal microflora reside in the gut of mammals where they are provided with an environment suitable for breeding and one which meets their nutritional needs. In return these microflora play a vital role in assisting the fermentation of carbohydrates and vitamin synthesis. Studies on Germ Free (GF) mice demonstrated that the intestinal microbiome plays a vital role in formation of mucosal immunity when compared with specific pathogen free animals (SPF). GF mice were shown to produce less IELs (Bandeira et al., 1990) and have a significantly reduced proportion of IgA-secreting plasma cells within lamina propria (Crabbé et al., 1968). There was also a marked decrease in Tregs (Ostman et al., 2006). A Study by Hooper et al. (2003) showed that GF mice have a markedly decreased level of Angiogenin 4 - which is a class of microbicidal proteins located in Paneth cells that are secreted into the gut lumen against microbes (Hooper et al., 2003). Peyer's patches of GF mice are also shown to have a smaller germinal centre than conventional mice (McDermott & Huffnagle, 2014). The germinal centre cells have surface IgA. These results are an indication that the gut microbiota are indeed paramount for the mucosal immunity. In the absence of microbial stimulation the intestinal immune systems is greatly underdeveloped (Hooper et al., 2003).
Factors affecting homeostasis of microbiota
Whilst the host attempts to maintain favourable microbiota by releasing specific factors such as mircoRNA’s and non-specific factors including antimicrobial peptides, mucus and IgA other factors such as age, diet and antibiotic usage can change the gut microbiota. Many studies have shown a link between disease and microbiota disorders. There are therapies which attempt to modulate this balance by using prebiotics, probiotics and faecal microbiota transplantation (Hasan & Yang, 2019).
Age and delivery pattern
Colonization of the intestinal microbes starts in utero by microbiota of the amniotic fluid and placenta (Collado et al., 2016). Method of delivery also affects early life gut microbiota development. Studies in humans have shown new-borns delivered vaginally have Lactobacillus and Prevotella derived from the mother's vaginal microbiota as their dominant primary gut microbiota where as those born via cesarean section acquire their gut microbiota from the skin resulting in Streptococcus, Corynebacterium, and Propionibacterium dominating (Dominguez-Bello et al., 2010; Mackie et al., 1999). By the age of 3 years these primary microbiota evolved and developed a greater diversity and stability and thus becoming more similar to an adult gut microbiota (Yatsunenko et al., 2012).
Diet
Infants raised on breast milk have a significantly different gut microbiota to those fed formula milk (Guaraldi & Salvatori, 2012). Numerous studies comparing this gut microbiota population showed that there is a difference in mucosal immune responses between breast fed and formula fed babies and that the breast fed group had a better immune response and a more stable microbial population (Grönlund et al., 2000;Bezirtzoglou et al., 2011) There is also a difference in dominating gut microbiota species when comparing adult humans who consume a vegetarian diet and those consuming a non-vegetarian diet (Walker et al., 2011).
Antibiotics
Antibiotics destroy both pathological and beneficial microbes indiscriminately causing dysbiosis and growth of unfavourable microbes (Klingensmith & Coopersmith, 2016). Studies have reported that clindamycin (Jernberg et al., 2007),clarithromycin and metronidazole (Jakobsson et al., 2010) and ciproflaxin (Dethlefsen & Relman, 2011) affect the microbiota structure for extended periods of time.
Methods of the gut microbiota modulation
Probiotics
Probiotics are living microorganisms which have been shown to promote growth of favourable gut microbes when taken at appropriate doses. They work by competing with harmful species for adhesion sites, or by production of antimicrobial compounds(Cleusix et al., 2007). For example, Stratiki et al (2007) described that adding Bidifobacter supplement to infant formula decreases intestinal permeability in preterm infants and reduces the abundance of fecal Bifidobacterium. Multiple systematic reviews analysing the role of probiotic on clinical outcomes showed that there was substantial evidence that probiotic supplementation had beneficial effects in urinary tract infections (Schwenger et al., 2015), chronic periodontitis (Ikram et al., 2018), necrotizing enterocolitis (Rees et al., 2017) and reduction of total cholesterol and low-density lipoproteins cholesterol (Wu et al., 2017). However, there is no clear agreement between researchers on the efficacy of probiotics in intestinal disease because differing doses and type of probiotics have been used in current studies (Ritchie & Romanuk, 2012).
Prebiotics
Prebiotics change the structure and activity of gut microbiota to benefit the host, they are usually composed of non-digestible carbohydrates, oligosaccharides or short polysaccharides such as inulin, oligofructose, galacto-oligosaccharides and xylo- oligosaccharides. A prebiotic must resist the gastric acids and be degraded and absorbed by digestive enzymes in the upper GI tract, they get fermented by gut microbiota and cause the growth or activation of useful species of gut microbiota (Quraishi et al., 2017), the main target species include Lactobacilli and Bifidobacteria (Tabibian et al., 2013). Research by Vandenplas et al. (2015) suggests that a mixture of galacto-oligosaccharides and fructo-oligosaccharides have the ability to increase Bifidobacteria and decrease Clostridium levels in the gut whilst galacto-oligosaccharides alone increase Lactobacillus (Vandenplas et al., 2015). Other studies showed arabino-xylooligosaccharides and inulin alter the intestinal barrier function and immune response (Van den Abbeele et al., 2018).
Faecal microbiota transplantation (FMT)
Faecal microbiota transplantation (FMT) is the administration of healthy donor faecal microbiota from healthy donors to patients with intestinal disease or dysbiosis of natural gut microbiota. FMT has been adapted into clinical practice for the treatment of Clostridium difficile infection where antibiotic treatment alone is unsuccessful (Khoruts & Sadowsky, 2016). There is a considerable list of other diseases of which FMT has shown to be effective including inflammatory bowel disease (IBD), irritable bowel syndrome, metabolic diseases, constipation, allergic disorders and many more (Hasan & Yang, 2019). There are also multiple pathways which can be used for transplantation of FMT including colonoscopy, enema (Kassam et al., 2012) and more recently capsules containing freeze dried faeces or bacteria and enteric-coated capsules have shown to have high efficiency of FMT (Tian et al., 2015).
Diseases caused by gut microbiome dysbiosis
Dysfunction of the intestinal immune system as a result of intestinal microbiota and mucosal immunity disturbance can lead to development of a multitude of diseases for example Inflammatory Bowel Disease (IBD) which causes intestinal inflammation and in turn triggers an abnormal immune response, examples of such IBD’s include Crohn’s disease (CD) and Ulcerative Colitis (UC). Studies have shown that genetic susceptibility alone is not enough to cause onset of IBD but that other factors such as environment and intestinal dysbiosis which causes an abnormal immune response and thus increases inflammation leading to destruction of the gastrointestinal tract (Seksik et al., 2003; Gophna et al., 2006). A study by Frank et al. (2007) showed decreased levels of commensal bacteria in IBD patients, namely Firmicutes and Bacteroidetes. Increased levels of detrimental bacteria, Proteobacteria and Actinobacteria were also seen. This reduction in microbial diversity hinders the ability of the microbiota to adapt to environmental changes and causes an impaired ability to adapt to natural disturbances. Inflammatory responses in IBD can be regulated by active bacterial products. For example, early onset IBD was shown to be linked to IL-10 deficiency (Glocker et al., 2009; Shah et al., 2012).
Conclusion
The balance between the host and its intestinal microbiome is an important part of mucosal homeostasis. The host has specific mechanisms which attempt to carefully control the microbiome in order to prevent undesired inflammation whilst simultaneously allowing for adequate microbial stimulation of the intestinal immune system to ensure proper development. Any disturbance in this microbial balance can lead to disruption of the mucosal immune system and lead to onset of inflammatory diseases. There are many cells and tissue types that are responsible for intestinal homeostasis, and they work together to prevent pathogen growth. The effector and induction sites in the host animal play a key role in colonisation resistance against pathogens. Targeted treatments that attempt to balance the gut microbiome are likely to become an effective method for addressing chronic inflammatory diseases.
References
Bandeira, A., Mota-Santos, T., Itohara, S., Degermann, S., Heusser, C., Tonegawa, S., & Coutinho, A. (1990). Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. The Journal of Experimental Medicine, 172(1), 239–244. https://doi.org/10.1084/jem.172.1.239
Bertics, P., J., Koziol-White, C. J., Gavala, M. L., & Wiepz, G. J. (2013). Middelton’s Allergy (Bd. 1). Bezirtzoglou, E., Tsiotsias, A., & Welling, G. W. (2011). Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe, 17(6), 478–482. https://doi.org/10.1016/j.anaerobe.2011.03.009
Cleusix, V., Lacroix, C., Vollenweider, S., Duboux, M., & Le Blay, G. (2007). Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiology, 12(7). https://doi.org/10.1186/1471-2180-7-101
Collado, M. C., Rautava, S., Aakko, J., Isolauri, E., & Salminen, S. (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports, 6(1), 23129. https://doi.org/10.1038/srep23129
Crabbé, P. A., Bazin, H., Eyssen, H., & Heremans, J. F. (1968). The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract—PubMed. https://pubmed.ncbi.nlm.nih.gov/4176641/
Dethlefsen, L., & Relman, D. A. (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences of the United States of America, 15(108). https://doi.org/10.1073/pnas.1000087107
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., & Knight, R. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences, 107(26), 11971–11975. https://doi.org/10.1073/pnas.1002601107
Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., & Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America, 104(34), 13780–13785. https://doi.org/10.1073/pnas.0706625104
Glocker, E.-O., Kotlarz, D., Boztug, K., Gertz, E. M., Schäffer, A. A., Noyan, F., Perro, M., Diestelhorst, J., Allroth, A., Murugan, D., Hätscher, N., Pfeifer, D., Sykora, K.-W., Sauer, M., Kreipe, H., Lacher, M., Nustede, R., Woellner, C., Baumann, U., … Klein, C. (2009). Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England Journal of Medicine, 361(21), 2033–2045. https://doi.org/10.1056/NEJMoa0907206
Gophna, U., Sommerfeld, K., Gophna, S., Doolittle, W. F., & Veldhuyzen van Zanten, S. J. O. (2006). Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis—PubMed. J Clin Microbiol, 44(11), 4126–4141.
Grönlund, M. M., Arvilommi, H., Kero, P., Lehtonen, O. P., & Isolauri, E. (2000). Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: A prospective follow up study of healthy infants aged 0-6 months. Archives of Disease in Childhood. Fetal and Neonatal Edition, 83(3), 186–192. https://doi.org/10.1136/fn.83.3.f186
Guaraldi, F., & Salvatori, G. (2012). Effect of breast and formula feeding on gut microbiota shaping in newborns. Frontiers in Cellular and Infection Microbiology, 2, 94. https://doi.org/10.3389/fcimb.2012.00094 Hasan, N., & Yang, H. (2019). Factors affecting the composition of the gut microbiota, and its modulation. PeerJ, 7. https://doi.org/10.7717/peerj.7502
Hooper, L. V., Stappenbeck, T. S., Hong, C. V., & Gordon, J. I. (2003). Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nature Immunology, 4(3), 269–273. https://doi.org/10.1038/ni888
Ikram, S., Hassan, N., Raffat, M. A., Mirza, S., & Akram, Z. (2018). Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. Journal of Investigative and Clinical Dentistry, 9(3). https://doi.org/10.1111/jicd.12338
Jakobsson, H. E., Jernberg, C., Andersson, A. F., Sjölund-Karlsson, M., Jansson, J. K., & Engstrand, L. (2010). Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PloS One, 5(3). https://doi.org/10.1371/journal.pone.0009836
Jernberg, C., Löfmark, S., Edlund, C., & Jansson, J. K. (2007). Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. The ISME Journal, 1(2), 56–66. https://doi.org/10.1038/ismej.2007.3
Kassam, Z., Hundal, R., Marshall, J. K., & Lee, C. H. (2012). Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Archives of Internal Medicine, 172(2), 191–193. https://doi.org/10.1001/archinte.172.2.191
Khoruts, A., & Sadowsky, M. J. (2016). Understanding the mechanisms of faecal microbiota transplantation. Nature Reviews. Gastroenterology & Hepatology, 13(9), 508–516. https://doi.org/10.1038/nrgastro.2016.98
Klingensmith, N. J., & Coopersmith, C. M. (2016). The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Critical Care Clinics, 32(2), 203–212. https://doi.org/10.1016/j.ccc.2015.11.004
Li, N., Shi, N., Duan, X., & Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system | SpringerLink. Springer. https://link.springer.com/article/10.1186/s40779-017-0122-9
Mackie, R. I., Sghir, A., & Gaskins, H. R. (1999). Developmental microbial ecology of the neonatal gastrointestinal tract. The American Journal of Clinical Nutrition, 69(5). https://doi.org/10.1093/ajcn/69.5.1035s
McDermott, A. J., & Huffnagle, G. (2014). The microbiome and regulation of mucosal immunity—PubMed. Immunology, 142(1), 24–31.
Ostman, S., Rask, C., Wold, A. E., Hultkrantz, S., & Telemo, E. (2006). Impaired regulatory T cell function in germ-free mice—PubMed. https://pubmed.ncbi.nlm.nih.gov/16897813/
Peng, J., Tang, Y., & Huang, Y. (2021). Gut health: The results of microbial and mucosal immune interactions in pigs. Animal Nutrition. https://doi.org/10.1016/j.aninu.2021.01.001
Quraishi, M. N., Sergeant, M., Kay, G., Iqbal, T., Chan, J., Constantinidou, C., Trivedi, P., Ferguson, J., Adams, D. H., Pallen, M., & Hirschfield, G. M. (2017). The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut, 66(2), 386–388. https://doi.org/10.1136/gutjnl-2016-311915
Rees, C. M., Hall, N. J., Fleming, P., & Eaton, S. (2017). Probiotics for the prevention of surgical necrotising enterocolitis: Systematic review and meta-analysis. BMJ Paediatrics Open, 1(1). https://doi.org/10.1136/bmjpo-2017-000066
Ritchie, M. L., & Romanuk, T. N. (2012). A meta-analysis of probiotic efficacy for gastrointestinal diseases. PloS One, 7(4). https://doi.org/10.1371/journal.pone.0034938
Schwenger, E. M., Tejani, A. M., & Loewen, P. S. (2015). Probiotics for preventing urinary tract infections in adults and children. The Cochrane Database of Systematic Reviews, 23(12). https://doi.org/10.1002/14651858.CD008772.pub2
Seksik, P., Rigottier-Gois, L., Gramet, G., Sutren, M., Pochart, P., Marteau, P., Jian, R., & Doré, J. (2003). Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut, 52(2), 237–242. https://doi.org/10.1136/gut.52.2.237
Shah, N., Kammermeier, J., Elawad, M., & Glocker, E.-O. (2012). Interleukin-10 and interleukin-10-receptor defects in inflammatory bowel disease. Current Allergy and Asthma Reports, 12(5), 373–379. https://doi.org/10.1007/s11882-012-0286-z
Stratiki, Z., Costalos, C., Sevastiadou, S., Kastanidou, O., Skouroliakou, M., Giakoumatou, A., & Petrohilou, V. (2007). The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Human Development, 83(9), 575–579. https://doi.org/10.1016/j.earlhumdev.2006.12.002
Tabibian, J. H., Talwalkar, J. A., & Lindor, K. D. (2013). Role of the Microbiota and Antibiotics in Primary Sclerosing Cholangitis. https://www.hindawi.com/journals/bmri/2013/389537/
Tian, H., Ding, C., Gong, J., Wei, Y., McFarland, L. V., & Li, N. (2015). Freeze-dried, Capsulized Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. Journal of Clinical Gastroenterology, 49(6), 537–538. https://doi.org/10.1097/MCG.0000000000000330
Tlaskalová-Hogenová, H., Stepánková, R., Hudcovic, T., Tucková, L., Cukrowska, B., Lodinová-Zádníková, R., Kozáková, H., Rossmann, P., Bártová, J., Sokol, D., Funda, D. P., Borovská, D., Reháková, Z., Sinkora, J., Hofman, J., Drastich, P., & Kokesová, A. (2004, mai). Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. PubMed. https://pubmed.ncbi.nlm.nih.gov/15158604/
Tokuhara, D., Kurashima, Y., Kamioka, M., Nakayama, T., Ernst, P., & Kiyono, H. (2019). A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergology International, 68(1), 17–25. https://doi.org/10.1016/j.alit.2018.09.004
Van den Abbeele, P., Taminiau, B., Pinheiro, I., Duysburgh, C., Jacobs, H., Pijls, L., & Marzorati, M. (2018). Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells. Journal of Agricultural and Food Chemistry, 66(5), 1121–1130. https://doi.org/10.1021/acs.jafc.7b04611
Vandenplas, Y., Zakharova, I., & Dmitrieva, Y. (2015). Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. The British Journal of Nutrition, 113(9), 1339–1344. https://doi.org/10.1017/S0007114515000823
Walker, A. W., Ince, J., Duncan, S. H., Webster, L. M., Holtrop, G., Ze, X., Brown, D., Stares, M. D., Scott, P., Bergerat, A., Louis, P., McIntosh, F., Johnstone, A. M., Lobley, G. E., Parkhill, J., & Flint, H. J. (2011). Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The ISME Journal, 5(2), 220–230. https://doi.org/10.1038/ismej.2010.118
Wu, Y., Zhang, Q., Ren, Y., & Ruan, Z. (2017). Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLOS ONE, 12(6). https://doi.org/10.1371/journal.pone.0178868
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., Magris, M., Hidalgo, G., Baldassano, R. N., Anokhin, A. P., Heath, A. C., Warner, B., Reeder, J., Kuczynski, J., Caporaso, J. G., Lozupone, C. A., Lauber, C., Clemente, J. C., Knights, D., … Gordon, J. I. (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222–227. https://doi.org/10.1038/nature11053
Figures
Figure 1: Chelakkot, C., Ghim, J. & Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 50, 103 (2018). https://doi.org/10.1038/s12276-018-0126-x, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0/>, via Wikimedia Commons
