Itt írjon a(z) Mito_Aging-ról/ről
The Role of Mitochondria in Aging
Abstract
To establish a basis on which our research builds, in our essay we will first discuss the role of mitochondria in the production of ROS in correspondence to the ROS theory Harman proposed in 1978. Aging refers to the biological degeneration of bodily functions in living beings over time, on which mitochondria have an influence. An important cell organ for respiration, it is known that the mitochondrion contributes to cellular aging according to the free radical (ROS) theory, the production of ROS can mutate mitochondria DNAs. The growing connection between basic biology of aging and the pathogenesis of age-related diseases revealed by modern researches on mitochondrial biology has seen a shift of emphasis from ROS production to other aspects of mitochondrial physiology, such as the activation of inflammasomes by mitochondiral DNA through intracellular and extracellular pathways resulting in inflammation of tissues and an immune response that may cause tissue damage, and mitohormetis that include mitochondrial uncoupling and the mitochondrial unfolded protein pathway. The remaining part of our essay will explore some of the most recent researches that contradict the ROS theory, the validity of which is challenged as a growing rapport of current studies refutes that, instead of damaging the mtDNA and proteins (leading to deterioration of cells, tissues and organs), reactive oxygen species have the opposite effect of evoking metabolic health and longevity through hermetic mechanisms including autophagy.
Contents
Structure and function of mitochondria
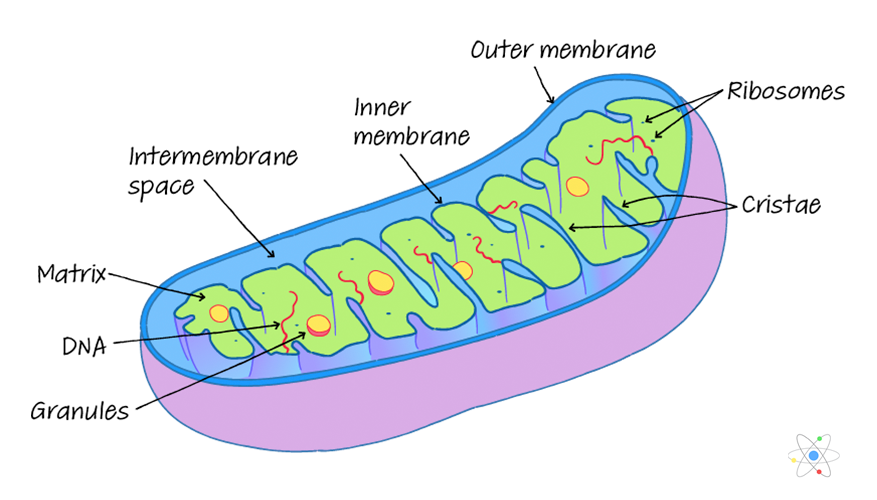
Mitochondria are the organ of cells in which most of the processes of oxygen breathing in eukaryotic organisms progress, and there is no mitochondria in prokaryotes. Mitochondria are composed of outer, inner, and substrate. (Two-middle membrane structure)(Figure 1) The inner lining of the mitochondria is wrinkled inward to form a Crista, which greatly expands the surface area of the inner membrane, allowing thousands of electronic transmitters per mitochondria. In other words, the more active cells are, the more mitochondria (e.g., muscle cells, liver cells) substrates are filled with liquid inside the inner membrane, and contain various enzymes, DNA, RNA, ribosomes, etc. In other words, it is possible to self-produce and synthesize proteins. The interior of the mitochondria is divided into two parts, the space between the membrane and the substrate. There is an electron transporter enzyme family in the endothelium, where the electron transfer process is followed, ATP is synthesized by ATP synthetic enzyme, and TCA circuits are conducted in the substrate.
|
’’’Figure 1.’’’ structure of mitochondria |
Mitochondria breathing and electronic transmission system
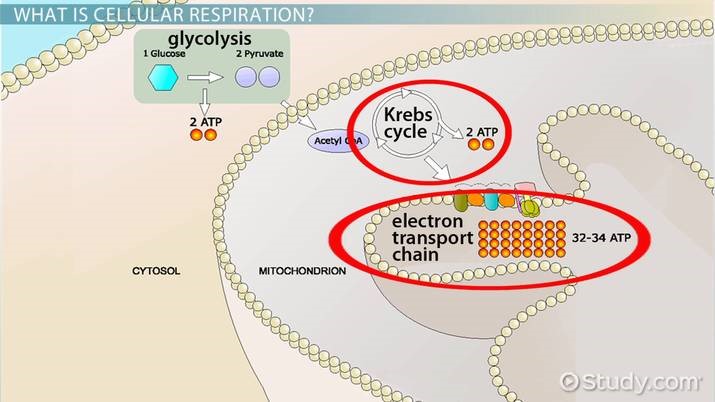
Mitochondria are energy plants with adequate structures for oxygen breathing in eukaryotic organisms, and a large amount of ATP is produced, especially in wrinkled inner membranes. The cellular breathing process consists of three stages of corresponding action, TCA cycle, and oxidative phosphorylation.(Figure 2)
i. glycolysis:
- The process of glucose disintegrating into pyruvic acid, a small molecule, because it cannot enter mitochondria: glucose entered into the cell is decomposed into the pyruvate 2 molecules through Glycosis, and pyruvic acid enters the mitochondria into the TCA circuit and completely breaks down into CO2 and water.
ii. TCA cycle:
- The process of organic matter being completely decomposed into inorganic matters: NADH and FADH2 are produced, which can generate large amounts of ATP through an electronic transmitter on the inside of mitochondria.
iii. Oxidizing:
- Process of mass synthesis of ATP: The amount of ATP generated by the decomposition of one molecule of glucose produces a total of 36-38 minutes of ATP, including the two molecules generated by the TCA circuit plus the 32-34 molecules produced by the electron transmitter.
The action is carried out in cytoplasm regardless of the presence or absence of oxygen, and the TCA circuitry and oxidative phosphorylation are conducted in the mitochondrial substrate and endothelium, respectively, when oxygen is sufficient. Glucose of one molecule is decomposed from cytoplasm to two molecules of pyruvic acid after the corresponding action, and if oxygen is present, it enters mitochondria and is completely decomposed into carbon dioxide and water after TCA circuits and oxidative phosphoric phosphoric acidization
|
’’’Figure 2.’’’ cell respiration |
The theory of reactive oxygen species
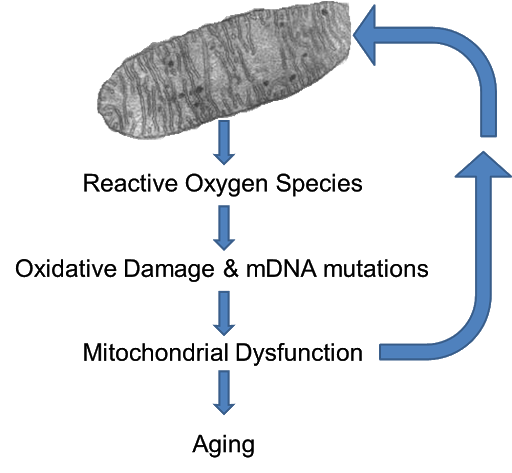
- The theory of reactive oxygen species was proposed by Harman in 1956, and the biomechanical components are subject to oxidative damage by a number of reactive oxygen species produced by the normal metabolic process, resulting in aging and death. Damage caused by free radicals is addressed by the ability of the living to defend themselves, but the defense is not 100% complete and is subject to harmful effects from some free radicals. The theory is that this harmful effect occurs slowly, in some cases, chronically throughout a lifetime or even years, which degrades the function of cells or tissues, which in turn causes disease and aging. The resulting phenomena do not appear in a short period of time, but over a long period of time, which is what we see as the aging process.(Figure 3)
|
’’’Figure 3.’’’ ROS theory |
i. ROS (reactive oxygen species)
- . Reactivate oxygen species is a common name for oxygen free radical and various oxygen compounds derived from it, all of which have highly reactive characteristics. Free radical means an atom or molecule with electrons that are not chemically paired to the outermost electron orbit. They are unstable because they lose electrons that do not make up the pair, or because they have the temperament of trying to get one more electron from the surroundings and go into a more stable state. Therefore, it is highly reactive because it easily reacts with surrounding compounds to lose or obtain electrons.
ii. Reactive oxygen formation in mitochondria
. The most important function of mitochondria in cells is ATP production. ATP production takes place in the respiratory chain and consists of 5 multi-subunits (multi-subunits; respiratory complexes I–IV, F1FO-ATP synthase) and two factors (cytochrome c [Cyt c] and coenzyme Q10). During this process, electrons are released from the mitochondrial reserve chain to produce superoxide by incompletely reducing oxygen molecules, and superoxide is converted to H2O2. About 1-2% of the oxygen needed for breathing is known to be incompletely reduced, generating superoxide. Superoxide is transformed into HO2 by copper/zinc superoxide disk (Cu/Zn-SOD) in the mitochondrial matrix or by copper/zinc superoxide disk in the intermembrane space (IMS). Many places where superoxide occurs in mitochondria are known, and the most important of them are the complex I and III of the respiratory chain.
iii. Damage to aging and dysfunction
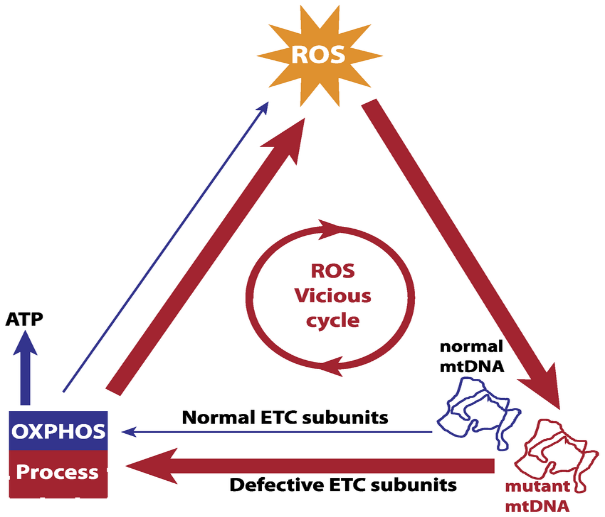
- Mitochondrial ROS vicious cycle.
- A putative positive feedback mechanism between mtDNA and ROS is based on the hypothesis that ROS-induced damaged mtDNA produce defective components of the ETC, thereby increasing electron leakage in the OXPHOS process and ROS production. The vicious cycle is expected to give an exponential expansion of mtDNA mutations over time, which eventually causes the loss of mitochondrial function in generating ATP.(Figure 4)
|
’’’Figure 4.’’’ ROS vicious cycle |
- Unbalanced ROS levels in the body act as the cause of various diseases including chronic inflammatory diseases, especially when the release of ROS occurs in mitochondria through ROS vicious cycle. Currently, it is known that cancer, aging processes, neurosurgery, atherosclerosis, and fibrosis are related to ROS.(Figure 5)
|
’’’Figure 5.’’’ mitochondrial dysfunction |
Aging is associated with the innate immune system
Inflammatory mediators
An activation of the innate immune system leads to a condition called inflammation. This inflammation is a result of work of inflammatory mediators, and the studies have shown that in elderly individual, the body exhibit higher circulating levels of inflammatory mediators. This relationship exist naturally, even without an age associated diseases such as cancer, cardiovascular disease, but in a normal aging. Looking at the measurements of the level of different inflammatory mediators, the data are most interesting with the level of circulating IL-6.
IL-6
- When IL-6 serum level elevates, this can predict incident disability, frailty, walking speed, and overall mortality.
Cytokine production
- Analyzing further suggested that basal levels of cytokine production have increased, but the invoked responses to a cytokine challenge have been reduced. This observation seem to be due to chronic activation of JAK-STAT signaling in the circulating immune cells of the aged patient.
IL-1 beta
- Increased level of another inflammatory mediator, IL-1 beta has shown to identify subset of elderly individuals with increased rate of hypertension, arterial stiffness, and more causes of mortality.
NLRP3
- Activation of NLRP3 inflammasome is observed in many age-related conditions; atherosclerosis, Alzheimer’s disease, and metabolic syndrome. An experiment in genetic deletion of NLRP3 was done on mice and it appeared to have reduced inflammation associated with aging and slowed the age-dependent incidence of insulin resistance, cognitive decline, and frailty.
Looking at the relationship between studied levels of inflammatory mediators and aging factors, an anti-inflammatory strategy appeared to reduce age-related disease/events.
Mitochondria and inflammation
- The immune system is able to sense and respond to tissue damage and perceive the release of intracellular molecules in a parallel sense to dangerous pathogens. This “danger theory” even argues that some molecules released from dying cells might contribute to the signals that trigger an immune response as well, this phenomenon is termed, damage-associated molecular patterns (DAMPs). There is increasing number evidence that mitochondria might play an important role in the inflammation. It can represent a potential immune-stimulating DAMPs. This supposed mitochondrial DAMPs include the released mitochondrial DNA (mtDNA), N-formyl peptides from the translation of mitochondrial-encoded protein, and some unique lipids and phospholipid constituted of inner mitochondrial membrane.
|
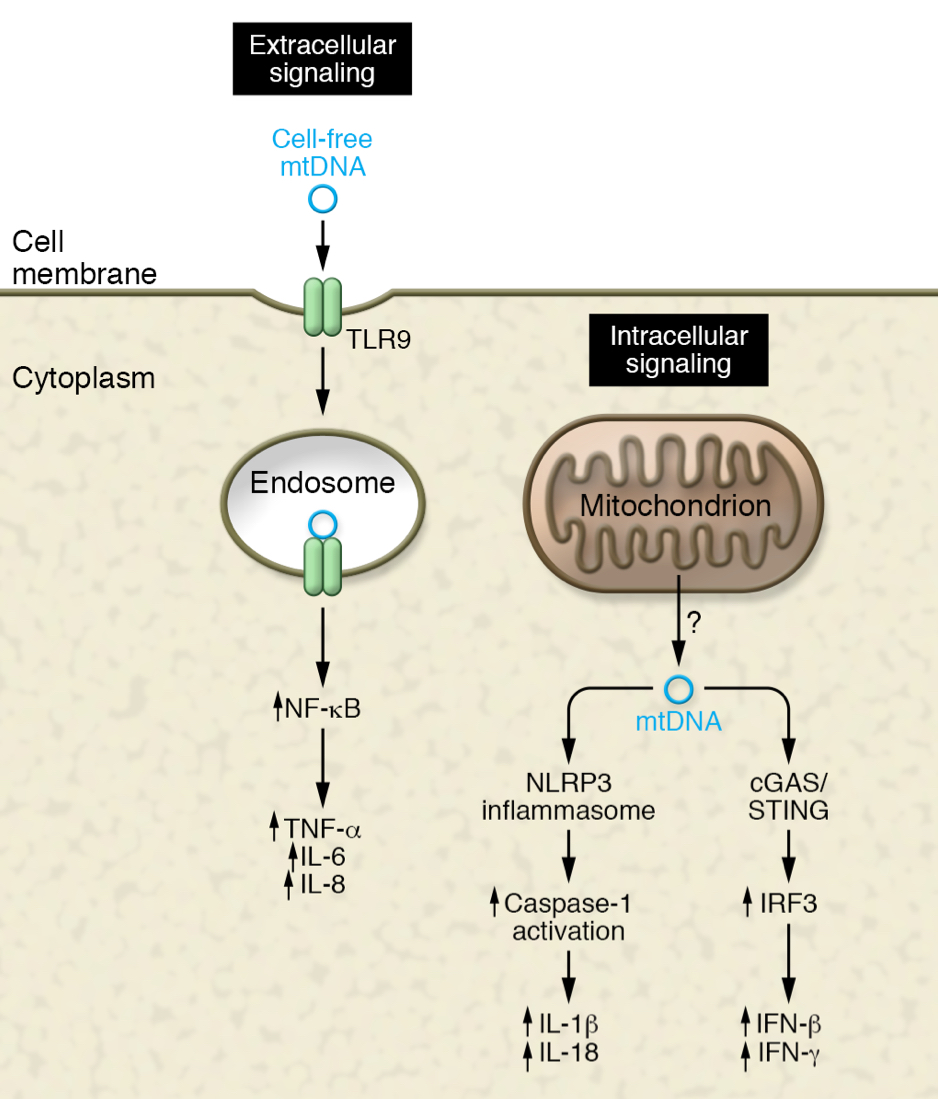
’’’Figure 6.’’’ mtDNA - inflammasome activation pathway |
I. Circulating mtDNA
Circulating mtDNA causes immune activation by activating the Toll-like receptor 9 (TLR90 signaling. In other hand, mitochondrial proteins activates by binding to formyl peptide receptor-1 (Figure 7). These activation were first observed in a case of trauma, where release of mtDNA and formyl peptides activated the circulating neutrophils to mediate tissue injury. More experiments were held to prove that mitochondrial DAMPs could re-demonstrate the neutrophil mediated organ injury followed by a traumatic event, without any tissue injury. Therefore, circulatory mtDNA could cause unnecessary inflammation. Circulating mtDNA is observed to increase with age after the fifth decade of life. DAMPs can also trigger the assemble of the inflammasome, as mentioned above, inflammasome activation is observed to identify the conditions of elderly individuals. And genetic inhibition of this pathway in mice has resulted to protect the animal from certain age related disorder. A center of work of inflammasome complexes is on NLRP3 due to its respond to wide range of stimuli. Stimulation of NLRP3 inflammasome causes activation of caspase-1, which activates potent cytokines (IL-1 beta and IL-18). An activation of caspase-1 can also induce mitochondiral damage. This is one of many relationship between inflammasomes and mitochondria. Further example can be the mitochondria-associated adaptor molecule, MAVS, can serve as a platform for inflammasome activation. And an evidence have been found for the mitochondrial phospholipid cardiolipin can directly bind and activate NLRP3.
ii. Intracellular mtDNA
How mtDNA is released to cytosol remains a bit mysterious. An understandable case is when massive trauma is involved, or other forms of cell death. Another possibility can be the release of mtDNA due to check by quality control mechanism.
Quality control mechanism
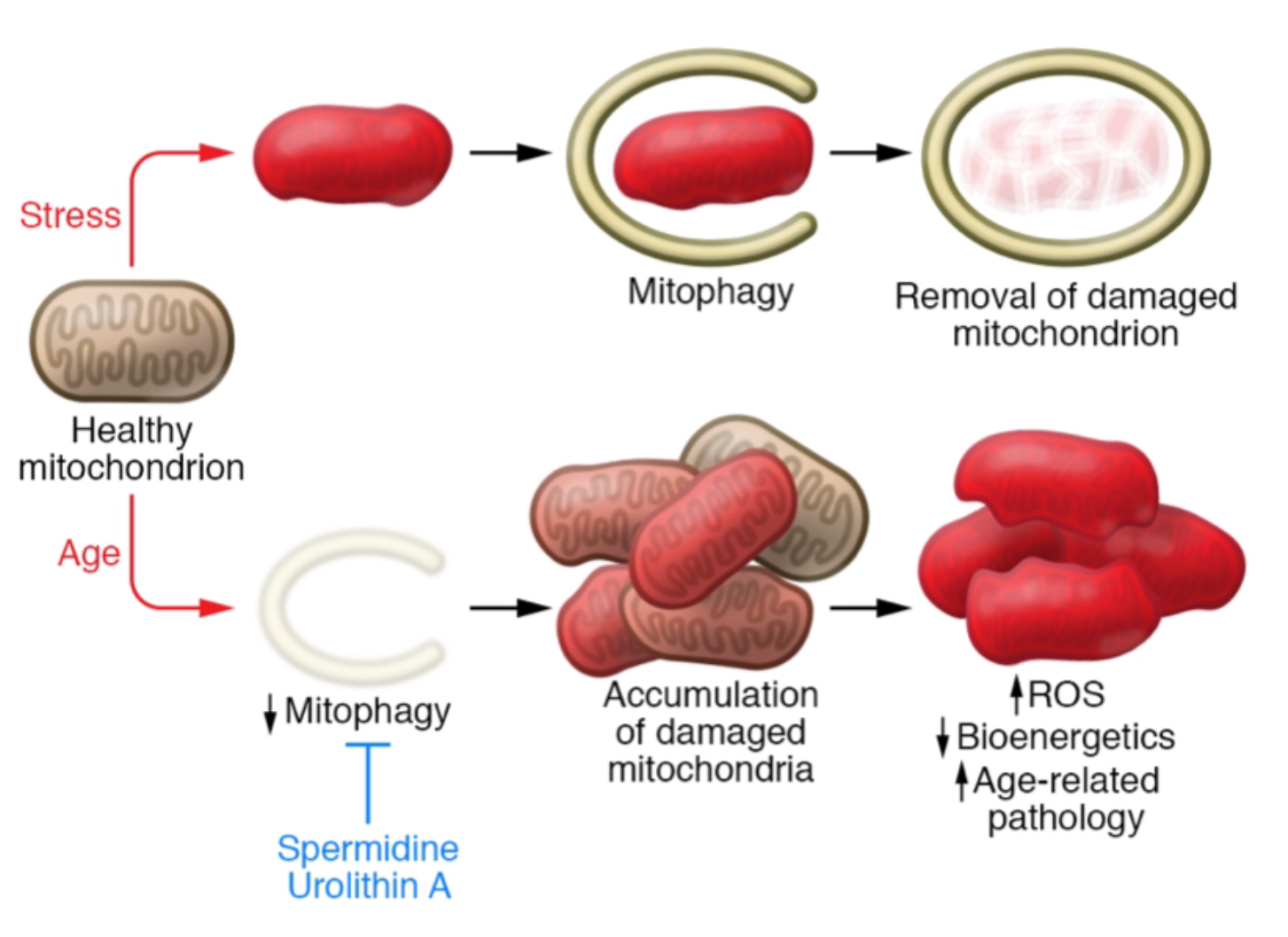
This work can particularly be held via autophagic/mitophagic removal of mal-functionaling mitochondria. Taking this mechanism into consideration, having disrupted autophagy can increase the number of free mtDNA in the cytosol and increase IL-1 beta secretion. There has been a discovery of evidence that in macrophages, mitophagy work to restrain the inflammasome activation by removing damaged mitochondria, which could cause an NLRP3 activation. This can decrease the inflammation causing from damaged mitochondria. Another report has shown that cytokine IL-10 can promote mitophagy by inhibiting the mTOR pathway. Some studies state that the level of mitophagy remarkably declines in mammalian tissues during normal aging. Autophagy does not only have intersection with the inflammasome activations, but also with the oxidative cycle. Specifically in mitophagy, its decline might fuel the vicious cycle of oxidative stress-induced, age-related tissue damage. (Figure 8) These studies have proven some intersection between inflammasome activation and mitophagy, and the prevention of age-related conditions.
|
’’’Figure 7.’’’ Aging causes mitophagic removal of stressed mitochondria. |
These mtDNA released into cytosol can activate TLR9 signaling and further cause inflammasome activation. Another pathway of immune system activation with mtDNA is being studied. This pathway considers mitochondria’s evolutionary origin, like foreign viral and bacterial DNA, mtDNA can also be recognized by cyclic GMP-AMP synthase (cGAS). This is a cytosolic sensor of double stranded DNA (dsDNA). Once the mtDNA is bound to dsDNA, this leads to the activation of the transcription factor IRF3. This activation causes induction of type 1 IFNs and IFN-stimulated nees, and this is a critical process for a response to viral infection. Intersection between this viral response pathway and mtDNA, leads to further studies of their relationship. An evidence has been found that infection with certain herpesviruses also caused decrease in mtDNA in cells. Both short-term, and long-term consequences of releasing mtDNA to antiviral defense have been evident. This mitochondrial initiated, virally triggered pathway might play an important ole in the associated inflammation observed in the elderly.
iii. Mitochondrial derived metabolites
There are other important ways that mitochondria could take part in the observed age-dependent inflammation. Tricarboxylic acid (TCA) metabolites is made from mitochondrial metabolism, and this appear to shape the immune response. A buildup of TCA metabolite succinate can result from metabolic shift, and a succinate-dependent HIF-a alpha induction can regulate IL-1 beta production. Through this regulation, immune response can be initiated , and also, combination of a high mitochondrial membrane potential along with succinate oxidation can lead to mitochondrial ROS production, producing a pro-inflammatory response. It is suggested that mitochondrial membrane potential might actually be an important determinant of immune responsiveness. Succinate appears to be one of many mitochondrial-derived metabolites that might modify the immune response and therefore contribute to the age-depending changes in immunity.
Mitohormesis
The mitochondrial stress response activated by a potentially damaging stimulus requires a coordinated dialogue with the cellular nucleus, known as mitonuclear communication. This interaction induced by the hormetic response in mitochondria increases lifespan and healthspan, particularly improving metabolism and immune system, in different animal models, from worms to mammals. Although multiple mediators and stress signals have been proposed to activate this protective mechanism, beneficial outcomes of mitohormesis are most probably due to an increase in mitochondrial ROS. Activation of other protective stress mechanisms as mitochondrial unfolded protein response or the increase in the expression of mitokines are also associated with the positive benefits exerted by mitohormesis. The complex balance between death and survival in the face of mitochondrial damage and dysfunction is mediated by the combative effects of mitohormesis through multiple mechanisms evolved.
i. Mitochondria and ROS Production
High levels of mitochondrial ROS production cause macromolecular damage[1], whereas low ROS levels may activate compensatory longevity-promoting signaling pathways. This is performed in enhanced systemic defense mechanisms by inducing an adaptive, hormetic response known as “mitohormesis”, where the induction of a reduced amount of mitochondrial stress leads to an increment in health and viability within a cell, tissue, or organism. Mild mitohormesis confers better stress resistance and life-span extension in various animal models, for instance, in fruit flies muscle mitohormesis preserves mitochondrial function – essential for muscular contraction and body movements - and promotes muscle longevity via systemic repression of insulin signaling.
A mainstream theory connects mitohormesis to mitochondrial uncoupling that participates in direct ROS mitigation and may extend lifespan by protecting cells from conditions that favour ROS production. Mitochondrial uncoupling refers to any biological process that dissociates electron transport from being used to drive mitochondria-dependent ATP synthesis. Mechanisms that allow protons to bypass the ATP synthase while entering the matrix essentially “short-circuit” the coupling of substrate oxidation to ADP phosphorylation. As mitochondria generate energy by creating a proton gradient across their inner membrane, some proteins of the inner mitochondrial membrane, such as the uncoupling proteins and the adenine translocator, can leak protons down the electrochemical gradient into the matrix and dissipate energy as heat while minimizing ROS production. This is deemed beneficial in the “uncoupling to survive” hypothesis (Papa and Skulachev, 1997; Brand, 2000), which postulates that the attenuation of ROS by partial uncoupling while maintaining sufficient ATP production is a potential mechanism for delaying cellular senescence[2], the condition of deterioration with age, when accompanied by deregulation of CA2+ -dependent signaling. This model contrasts with the idea first put forth to explain the rate-of-living model (and currently sometimes used to explain the effects of dietary restriction) that a low metabolic rate should confer a long lifespan. Instead, it argues that mild uncoupling will decrease ROS production and thereby extend lifespan even if it increases the “rate of living”. This is confirmed in Speakman’s testing in 2004 that found individual mice in the highest quartile of metabolic intensity lived 36% longer, displayed higher resting oxygen consumption rate and a higher rate of proton leak in skeletal muscle than those in the lowest quartile.
As mild uncoupling of mitochondrial respiration has been implicated in life-span extension with its excitatory effect on metabolic rate and inhibitory modulation of ROS production, its activation mechanism are even used to treat diseases such as obesity, cardiovascular diseases or neurological disorders.
ii. Unfolded Mitochondrial Protein Response (UPRmt)
Aging is associated with increased protein oxidative damage and an imbalance in proteostasis, which refers to the competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking and degradation of proteins present within and outside the cell. An imbalance in proteostasis is prompted by an impaired ubiquitin proteasome system, which is the principal mechanism for protein degradation that controls the break-up of cellular proteins by intracellular proteolysis. This produces the outcome of accumulation of misfolded proteins within the mitochondrial matrix, which is curbed by a mitochondrial-specific unfolded protein response (UPRmt) to restore proteostasis equilibrium by the increased secretion of chaperones and proteases.
The mitochondrial unfolded protein response (UPRmt) is an integral part of cellular mitohormesis to fulfil the major biomedical goal of identifying and therapeutically manipulating inherent programmes protecting against mitochondrial dysfunction. As an adaptive transcriptional response, UPRmt employs a retrograde signaling pathway that utilises the mitochondria-to-nucleus communication network to alleviate proteotoxic stress incited by the accumulation of misfolded or damaged mitochondrial-targeted proteins as well as to detect defects in mitochondrial translation. For instance, an excessive amount of oxidative phosphorylation (OXPHOS) complex constituents generating harmful sub-complexes will consequentially lead to loss of membrane potential, or ROS production as resultant from oxidative stress. A plausible cause of these mitochondrial perturbations can be attributed to the linkage between the accumulation of non-assembled OXPHOS subunits and defects in mitochondrial translation. In response, the UPRmt pathway is activated as a first line of defense against mitochondrial stresses in order to stabilise mitochondrial function, metabolic adaptations and innate immunity.
During UPRmt activation, ATFS-1 accumulated in the cytosol is directed by the nuclear localization signal released by the UPRmt to relocalise to the nucleus where it acts as a transcriptional regulator[3], instead of undergoing constitutive degradation by AAA+ -matrix protease LON after import to the mitochondrial matrix under standard physiological conditions. This allows ATFS-1 as a transcription factor to promote gene expression for protease transcription beneficial to the repair and recovery of the mitochondrial network caused by mutations in respiratory chain genes, and ultimately contribute to cell survival and organismal health. In this context, as Lon protease has a crucial enzymatic role in the degradation of oxidized proteins within the mitochondrial matrix, studies in mice suggest that Lon protease downregulation results in a compromised capacity of the aging mitochondrial in response to acute stress. In light of the overall interplay, it is widely regarded that UPRmt is regulated by the mitochondrial import efficiency of the transcription factor ATFS-1 in ringworms and potentially orthologous transcription factors ATF4, ATF5 and CHOP in mammals via similar mechanisms.
The surge in mitochondrial chaperone level during stress, including the matrix-localized chaperones Hsp60 and mtHsp70, promotes the folding of recoverable proteins, while the growth in proteases including the i-AAA and m-AAA proteases eliminates proteins that fail to fold or assemble. Moreover, the UPRmt includes a variety of anti-oxidant genes, such as mitochondrial superoxide dismutase and genes involved in glutathione metabolism, that restrict the protein and membrane perturbations brought about by ROS emitted from defective respiratory chains. More intensive transcription of protein homeostasis and anti-oxidant genes potentially stabilises the protein-folding environment to facilitate organelle function but also prepares for the regeneration of those salvageable organelles while irreparable organelles are degraded via mitophagy.
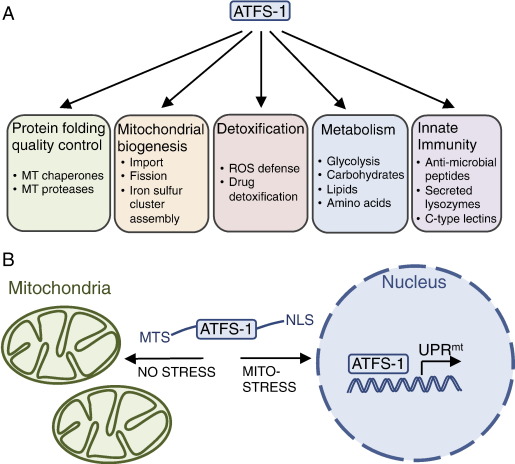
Although the UPRmt was initially documented in mammalian cell culture to mediate the negative effects of depleted mtDNA and accumulated misfolded proteins within the mitochondrial matrix by increasing mitochondrial chaperone level and proteastasis, the existence of a mechanism that detects mitochondrial dysfunction and identifies more than 400 genes induced during mitochondrial dysfunction is recently discovered in roundworms (Refer to Figure A below).
|
’’’Figure A.’’’ In response to mitochondrial damage or stress ATFS-1 induces genes involved in mitochondrial repair mechanisms including protein folding and protein quality control, as well as those involved in mitochondrial biogenesis, the detoxification response, metabolism and innate immune gene transcription. |
’’’Figure B.’’’ The UPRmt is activated during conditions such as mtDNA depletion, respiratory chain dysfunction, increased ROS or increased mitochondrial unfolded proteins, which is regulated by the mitochondrial protein-import efficiency of the transcription factor ATFS-1. In the absence of stress, ATFS-1 localizes to mitochondria via its mitochondria targeting signal (MTS), where it is degraded by the protease Lon. However, during mitochondrial dysfunction or stress, general mitochondrial protein import is attenuated, leading to the accumulation of a portion of ATFS-1 in the cytosol, followed by its translocation to the nucleus via its nuclear localization signal (NLS). In the nucleus, ATFS-1 induces the mitochondrial protective genes described in panel A, which promote survival and recovery from mitochondrial stress. |
Albeit the positive correlation between longevity and stress resistance as mitochondrial dysfunction contributes to normal aging as well as multiple devastating diseases, the demonstration that moderate mitochondrial perturbation extends lifespan by an impressive margin as large as up to 50% in nematodes was still surprising. The implication of UPRmt in life-span extension in worms, flies and mice also indicates its conserved role in the long-term maintenance of cellular homeostasis.
Nonetheless, despite the compensatory power of UPRmt in mitigating both positive and negative influence from dysfunctional mitochondria and lengthening lifespan, an inevitable detrimental cost is consistently attached to the parameters of development, animal size and fecundity: respiratory chain inhibition by UPRmt has dose-dependent effects on longevity which negatively correlate with developmental rate, fertility and animal size. This “mitochondrial threshold effect” suggests that up to a certain point mitochondria are able to function under compromised conditions and compensate for respiratory chain deficiencies. But exceeding beyond this threshold, compensatory pathways may not be able to offset the severe decline in mitochondrial function, which has fatal consequences. Increasing amounts of ROS may cause longevity only up to a certain point, after which the damage becomes harmful. In addition, the role of the UPRmt in the enhanced longevity conferred by modest mitochondrial dysfunction during exposure to mitochondrial stress occurs only in development, but not adulthood. Intriguingly, exposure to mitochondrial stress increased lifespan only if it occurred during development, but not during adulthood as well.
Conclusion
The components of mitochondria, most extensively known as the powerhouse of cells, share a complex relationship with the biological phenomenon of aging as an instrumental contributive factor. As neoteric researches have begun to follow a doubtful approach in scrutinising previously accepted hypotheses on the trajectory to understanding the intricate functioning of the cell organelle, more scientific evidence are expected to emerge in support of or dispute against the Harman’s theory.
References
1. Adriaensen, W., Matheï, C., Vaes, B., van Pottelbergh, G., Wallemacq, P. and Degryse, J., (2015): Interleukin-6 as a first-rated serum inflammatory marker to predict mortality and hospitalization in the oldest old: A regression and CART approach in the BELFRAIL study. Experimental Gerontology, (69), pp.53-61.
2. Callegari, S. and Dennerlein, S., (2018): Sensing the Stress: A Role for the UPRmt and UPRam in the Quality Control of Mitochondria. Frontiers in Cell and Developmental Biology, (6), pp.12.
3. Chen, Q., Vazquez, E., Moghaddas, S., Hoppel, C. and Lesnefsky, E., (2003): Production of Reactive Oxygen Species by Mitochondria. Journal of Biological Chemistry, 278(38), pp.36027-36031.
4. Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, (2017): Mitochondrial Dysfunction and Redox Imbalance as a Diagnostic Marker of “Free Radical Diseases”. 37(10).
5. Ferrucci, L., Penninx, B., Volpato, S., Harris, T., Bandeen-Roche, K., Balfour, J., Leveille, S., Fried, L. and Md, J., (2002):Change in Muscle Strength Explains Accelerated Decline of Physical Function in Older Women With High Interleukin-6 Serum Levels. Journal of the American Geriatrics Society, 50(12), pp.1947-1954.
6. Goldberg, E. and Dixit, V., (2015): Drivers of age-related inflammation and strategies for healthspan extension. Immunological Reviews, 265(1), pp.63-74.
7. Harman, D., (1956):Aging: A Theory Based on Free Radical and Radiation Chemistry. Journal of Gerontology, 11(3), pp.298-300.
8. Iyer, S., He, Q., Janczy, J., Elliott, E., Zhong, Z., Olivier, A., Sadler, J., Knepper-Adrian, V., Han, R., Qiao, L., Eisenbarth, S., Nauseef, W., Cassel, S. and Sutterwala, F., (2013): Mitochondrial Cardiolipin Is Required for Nlrp3 Inflammasome Activation. Immunity, 39(2), pp.311-323.
9. Jang, J., Blum, A., Liu, J. and Finkel, T., (2018): The role of mitochondria in aging. Journal of Clinical Investigation, 128(9), pp.3662-3670.
10. Gonzalez-Freire, M., de Cabo, R., Bernier, M., Sollott, S., Fabbri, E., Navas, P. and Ferrucci, L., (2015): Reconsidering the Role of Mitochondria in Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 70(11), pp.1334-1342.
11. CEDIKOVA, M., PITULE, P., KRIPNEROVA, M., MARKOVA, M. and KUNCOVA, J., (2016): Multiple Roles of Mitochondria in Aging Processes. Physiological Research, (3) pp.S519-S531.
12. Mookerjee, S., Divakaruni, A., Jastroch, M. and Brand, M., (2010): Mitochondrial uncoupling and lifespan. Mechanisms of Ageing and Development, 131(7-8), pp.463-472.
13. Pinti, M., Cevenini, E., Nasi, M., De Biasi, S., Salvioli, S., Monti, D., Benatti, S., Gibellini, L., Cotichini, R., Stazi, M., Trenti, T., Franceschi, C. and Cossarizza, A., (2014): Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. European Journal of Immunology, 44(5), pp.1552-1562.
14. Schulz, A. and Haynes, C., (2015): UPRmt-mediated cytoprotection and organismal aging. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1847(11), pp.1448-1456.
15. Soysal, P., Stubbs, B., Lucato, P., Luchini, C., Solmi, M., Peluso, R., Sergi, G., Isik, A., Manzato, E., Maggi, S., Maggio, M., Prina, A., Cosco, T., Wu, Y. and Veronese, N., (2016): Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Research Reviews, (31), pp.1-8.
16. Lombard DB, Chua KF et al. (2013): “Theories on Aging.” Theories on Aging, Boston University School of Public Health (1) 1.
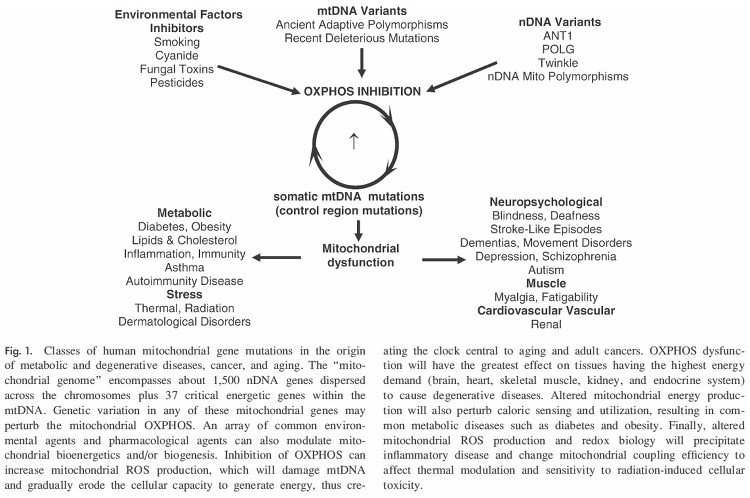
17. Wallace, D.C. (2005): A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annual Review of Genetics, 39(1), pp.359–407.
18. Yi, H.-S., Chang, J.Y. and Shong, M. (2018): The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases. Journal of Molecular Endocrinology, 61(3), pp.R91–R105.
19. Youm, Y.-H., Grant, R.W., MC Cabe L.R., Albarado, D.C., Nguyen, K.Y., Ravussin, A., Pistell, P., Newman, S., Carter, R., Laque, A., Münzberg, H., Rosen, C.J., Ingram, D.K., Salbaum, J.M. and Dixit, V.D. (2013): Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metabolism, 18(4), pp.519–532.
20. Yoo, Y.D. (2013): Mitochondrial Reactive Oxygen Species Production Mediated by Romo1 Expression. Hanyang Medical Reviews, 33(2), p.90.
21. Poovathingal, S.K., Gruber, J., Halliwell, B. and Gunawan, R. (2009): Stochastic Drift in Mitochondrial DNA Point Mutations: A Novel Perspective Ex Silico. PLoS Computational Biology, 5(11), p.e1000572.
22. Wallace, D.C. (2010): Mitochondrial DNA mutations in disease and aging. Environmental and Molecular Mutagenesis,(2) 440–50
Figures
Figure 1. Structure of mitochondria from Markgraf, Bert. "Mitochondria: Definition, Structure & Function (with Diagram)" sciencing.com, https://sciencing.com/mitochondria-definition-structure-function-with-diagram-13717287.html. 8 May 2020.
Figure 2. Cell respiration from https://study.com/academy/lesson/cellular-respiration-in-mitochondria.html
Figure 3. Ros theory from http://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH/Aging/mobile_pages/Aging3.html
Figure 4. Viscous cycle from https://journals.plos.org/ploscompbiol/article/figure/image?size=large&id=10.1371/journal.pcbi.1000572.g001
Figure 5. Mitochondrial dysfunction from Wallace DC., Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagen. 2010 Jun;51(5):440-50.
Figure 6 and 7. The role of mitochondria in aging. 2020. Authors own. Adapted from Arnon et al, (2018)
Figure A. ATFS-1's regulatory role in a broad transcriptional programme. 2020. Authors own. Adapted from Schulz and Hayne, (2015)
Figure B. Activation of UPRmt. 2020, Authors own. Adapted from Schulz and Haynes, (2015)
[1] which is why superoxide is broken down as they are generated by 3 superoxide dismutase isozymes that form a powerful ROS-scavenging/antioxidant system to avoid excessive ROS toxicity: the cytosolic SOD1 (Cu2+Zn2+-SOD), mitochondrial SOD2 (Mn2+-SOD), and extracellular SOD3. Deficiency of SOD1 and SOD2 leads to oxidative stress in mice; SOD1 deficiency accelerates sarcopenia, a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength.
[2] Mitochondrial superoxide production increases with replicative age in cultured human fibroblasts and is considered as a major contributing factor to replicative senescence.
[3] ATFS-1 is a regulatory substance that controls the expression of 500 genes that impact several cellular processes including immune regulators (e.g. antibacterial-related factor peptide 2 (Abf-2), metabolic enzymes (e.g. glutaminase) and additional transcriptional factors.