Neuroscience of Motivation
Authors: Andrea M Cini Bruno, Ann Mariya and Lily Molnar
Supervisor: Prof. Istvan Toth
Department of Physiology, University of Veterinary Medicine, Budapest
Motivation, in recent times, has been defined as a set of processes through which organisms regulate the probability, proximity and availability of stimuli. Each motivated action is the result of a number of biological and psychological processes that have been developed with experience and through evolution. Behaviour is directed towards or away from a particular stimuli, as well as actions that involve interacting with those stimuli.The main purpose of motivation is that it gives structure to most of the observable features of behaviour (Salamone & Correa, 2015).
Motivated behaviour typically has different phases. The terminal stage of motivated behaviour is the direct interaction with the goal stimuli, this is commonly referred to as the consummatory phase. The phases that engage in behaviour which gain access to these stimuli or makes the occurrence more likely are called “appetitive”, “preparatory” , or “seeking” phases (Salamone & Correa, 2012).
Behavioural action, however, may be associated with a number of costs including physical and mental effort, time, discomfort and danger. However, a number of benefits are also associated, including the fulfillment of physiological and psychosocial needs, and escape from harmful situations (Simpson & Balsam, 2015). Effort related capabilities are highly adaptive and in the natural environment, survival of an organism depends upon the extent to which it can overcome time-effort related response costs to obtain the stimuli, which is an important determinant of an organism’s choice of behavior (Salamone & Correa, 2015).
Contents
Neuroanatomy of Motivation
Dopamine (DA) neurons are called “reward” neurons, but this is often overgeneralized (Salamone & Correa, 2012). Progressive ratio breakpoints and self stimulation reward thresholds are behavioural measures used in experiments to mark the “reward” function of dopamine (DA). But recently it has been proved that these measures reflect processes involving exertion of effort, perception of opportunity costs, and decision making (Hernandez et al., 2010).These trends have all contributed to changing the dopaminergic involvement in motivation.
Dopamine
Dopamine is a key neuromodulator responsible for motivation-cognition interactions. Motivation influences a broad range of cognitive processes, i.e., attention, learning, memory and perception (Salamone & Correa, 2012).
Dopamine is produced in the ventral tegmental area (VTA) and substantia nigra located within the midbrain. The long axons emerging from these centres extend to other parts of the brain i.e., nucleus accumbens, dorsal striatum and prefrontal cortex (Matsuda et al., 2009).
Importance of Dopamine
The thinking process is quite effortful, one avoids doing demanding activities even if valuable goals could be achieved; as this requires an extended allocation of working memory for cognitive control. Working memory capacity is severely limited for cognitive control. Optimizing memory allocation is therefore critical to optimizing behaviour. (Kurzban et al., 2013).
The midbrain dopamine system translates cost- benefit information into adaptive working memory allocation, therefore optimizing working memory allocation. DA is critical for action selection. DA supports value learning and decision making about cognitive actions, thus resulting in behaviours that maximise reward with respect to effort (Westbrook & Braver, 2016).
DA Pathways
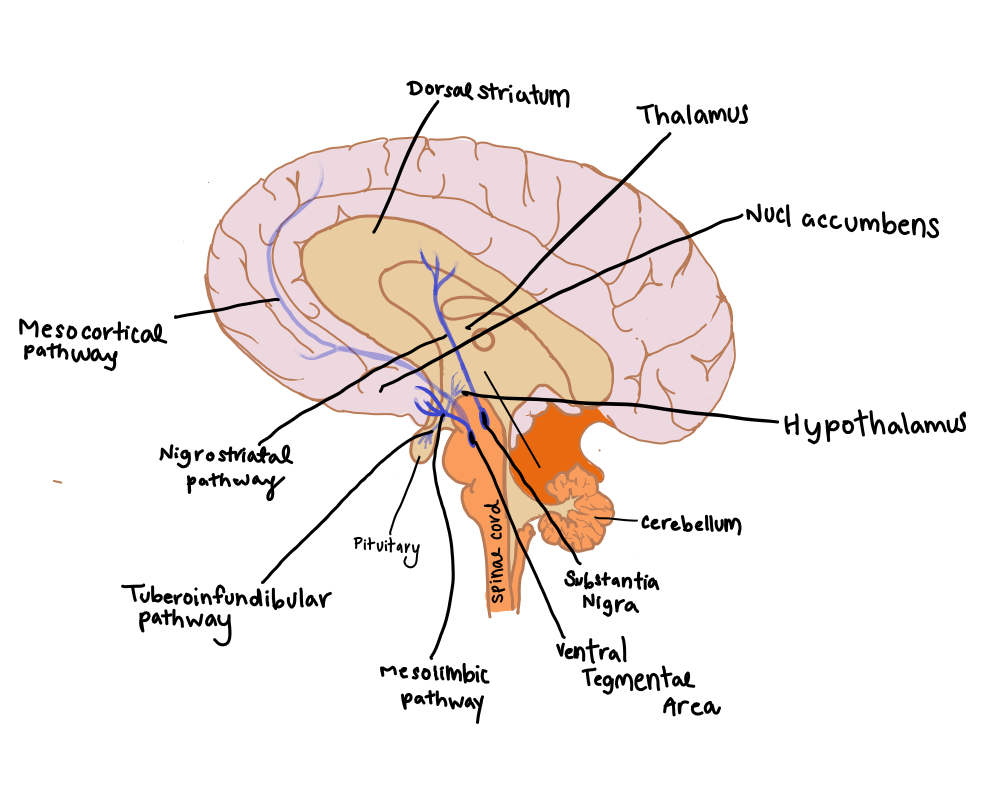
|
Figure 1 Dopaminergic pathways of the neural system (Adapted from Lynch, 2015) |
Mesocortical pathway- projects DA to multiple cortical areas (entire cortical mantle for primates) from the VTA. This pathway regulates important aspects of reward processing, comparative valuation and option assessment (Haber & Knutson, 2010) (Fig 1).
Mesolimbic pathway – transports dopamine from the VTA to the ventro-medial striatum (location of nucleus accumbens) from where it is transported to amygdala, hippocampus, prefrontal cortex (mesocortical pathway). The amygdala and hippocampus are associated with emotion and memory formation, respectively (Haber & Knutson, 2010) (Fig 1).
Nigrostriatal pathway - originates in the zona compacta of the substantia nigra and projects to the dorsal striatum. The nigrostriatal DA system has been associated in the control of procedural aspects of movement and motivated behaviors. DA release in the dorsal striatum reflects motor activation (Hull & Rodríguez-Manzo, 2017) (Fig 1 ).
Dopamine Dynamics
Dopaminergic signals project from VTA to the nucleus accumbens. These signals are phasic, so, by modifying synaptic strengths, these signals can help adjust reward expectations (Yagishita et al., 2014). The release of dopamine from VTA promotes learning by prediction errors. During conditioned learning, the activity of VTA dopamine neurons change from being based on reward- reception stimulus to reward-predicting stimulus and, in this way, promotes learning by prediction errors (Glimcher, 2011). On the other hand, local release of DA by nucleus accumbens anticipates and maintains rewards (Steinberg et al., 2013).
Mohebi et al has recorded neural activity and DA release in rats moving freely in a behavioral task (Bandit Task). In the Bandit Task, the rats push their nose into a central port on a light signal, after which, upon hearing an audible signal, they push their nose into an adjacent port and receive a food reward through a separate port. As the reward rate increased, the extracellular dopamine levels gradually increased ( Mohebi et al., 2019). However, there was no increase in the activity of dopaminergic neurons in the VTA. Therefore, the release of DA in the nucleus accumbens in the absence of increased neuronal activity proves that the two are dissociated. It can be concluded that the local regulation of DA release in the accumbens is independent of the activity of dopaminergic neurons in VTA ( Mohebi et al., 2019).
Introduction to Accumbens
The motivational process interacts with mechanisms related to emotions, learning etc., and there is a considerable overlap between these functions. Therefore, a point-to-point mapping of the process is indivisible, though some dopaminergic manipulations can help understand specific aspects of motivation, motor function or learning (Salamone & Correa, 2012). Hence to understand different aspects of motivation, manipulations of accumbens DA are devised. The effects of interference are highly selective, impairing some aspects while leaving others intact (Salamone & Correa, 2012).
Accumbens DA – Effort Related Choice
Effort based decisions are studied using tasks that offer a choice between a high effort task with a high value reinforcer and low effort task with a low value reinforcer. One such experiment offered rats a choice between fixed ratio 5 (FR5) lever pressing (to obtain a preferred carbohydrate pellets), or to consume less preferred chow. Under control conditions, rats pressed on the FR5 schedule to get most of their food and sparingly ate the chow. When a DA antagonist is administered the lever pressing is decreased and the rats showed a compensatory reallocation of behaviour and selected a new path (to consume chow) (Salamone, 1991). Randall reported that the administration of bupropion (catecholamine uptake inhibitor which elevates extracellular DA and increases expression of phosphorylated DARPP-32 in a manner consistent with increased D1 and D2 signalling) substantially increased the selection of high effort action in rats (Randall et al, 2015).
Neurobiology of Motivational Drives
Neurobiology is the study of nerve cells and the organisation of such cells into functioning units capable of processing information and mediating behaviour (Shepherd, 1988). The neurobiological mechanisms associated with interactions of the motivation systems are of relative importance, however they are still not well understood (Simpson & Balsam, 2015).
Motivation to Feed
Food can be consumed both for energy and pleasure. Rather than being an unconditional reflex that occurs when one is hungry it has been concluded to also be influenced by a diverse number of factors, including homeostatic signals, food availability and the amount of energy-rich compounds circulating through the system. Food consumption may also be the result of non-homeostatic signals that convey information with regard to stress, social situations and other factors. The identification of links between homeostatic and non-homeostatic influences has added to the complexity associated with the neural foundation of the motivation to eat (Woods & Begg, 2015). With the increase of food palpability, studies indicate that the homeostatic circuits (those influenced by adiposity and satiation signals) have become overshadowed by the motivation to eat excessive amounts of food for its rewarding value. This situation is aggravated by novel distal cues in the form of advertisements that specifically appeal to pleasure-based and other non-homeostatic motivations to eat. Furthermore, the reliability of more traditionally distal cues with regard to caloric content, gustation, and smell have become less reliable due to the use of artificial sweeteners and fats which, in turn, influence proximal cues used in the determination of portion size and results in over eating (Woods 1991, 2009). The uptake of food has been defined as a biological stress (Woods 1991) and, due to the complexity and intersection of meals and homeostatic, hedonic and emotional factors they may be associated with a stress response in certain individuals which may induce, in others, eating disorders such as anorexia nervosa (Woods & Begg, 2015).
Sexual Motivation
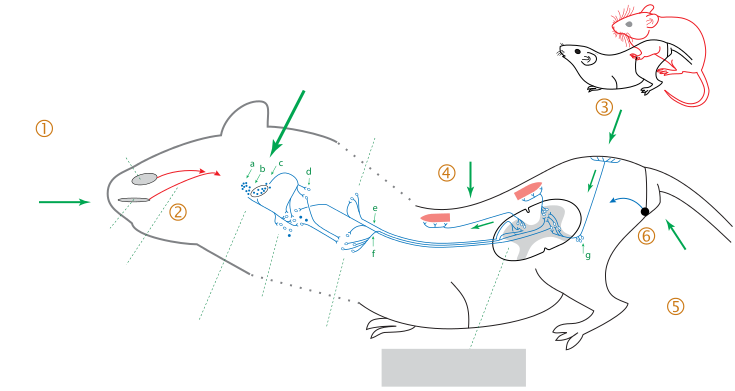
|
Figure 2 Lordosis Stimulation in rats (Castel, 2014) |
The key hormone behind sexual motivation is the steroid hormone oestradiol. This hormone is known to be driven by a number of non-homeostatic mechanisms. During experimentation on laboratory rodents, the stimulus (male mounting) and reaction (female’s reaction) was studied. The response of a female to male mounting depended on the activation of the hypothalamus by oestradiol. If the hypothalamus had not been sufficiently exposed, the female’s reaction to mounting was to reject the male. However, studies have shown that if her ventromedial hypothalamus neurons had been sufficiently exposed to estrogens, in combination with progesterone, the female’s reaction to mounting was lordosis (dorsiflexion of the vertebral column(Fig 2)), an essential posture for successful intercourse (Pfaff & Schwartz-Giblin 1988). Irrespective of oestrogen levels, males mounting causes somatosensory signals to be sent from the skin on the flank and rump to the spinal cord via ascending pathways to the medulla and the midbrain. For lordosis to occur from this point, an oestrogen-dependent signal from the ventromedial hypothalamus must also have reached the midbrain. If this has occurred, the midbrain influences the medullary reticular neurons and their subsequent facilitation of action on the axial muscle motor neurons in the spinal cord will cause lordosis (Magarinos & Pfaff 2015).
Motivation Drive for Social Behaviour
Motivation with respect to social behaviours is influenced primarily by past experiences and the internal state as well as on the behaviour of other animals. Over a range of species, oxytocin and vasopressin systems have been linked to the regulation of motivated social behaviours. These are fundamental neurochemical signals that act throughout the brain with the scope of influencing socially motivated behaviour. Studies have shown that these hormones influence both cooperative and competitive behaviours through their actions within the social behaviour neural network (section of the brain controlling social behaviour (Newman,1999; Caldwell & Albers, 2015). It has also indicated that the mesolimbic dopamine system is also involved in the regulation of cooperative and competitive behaviour with the two systems being categorised as a larger social decision-making network. Although further research is still required for the thorough understanding of the neurochemical linkages, it has been proposed that oxytocin and vasopressin may link together specific sections of the social behaviour and neural network and the mesolimbic dopamine system to initiate social behaviour drives. Evidence indicates that these hormones act on the structure making up the mesolimbic dopamine system which, upon its manipulation, may influence the same social behaviours as oxytocin and vasopressin (Caldwell & Albers, 2015).
Neural Abnormalities in Motivation
Motivation Deficit
Sometimes, the body loses its ability to regulate signals for motivation. The lack of motivation is called avolition. Avolition is when one is unable to look forward to pleasurable outcomes, and can be a symptom of Schizophrenia. Typically animals will see a neutral stimulus and gain attraction through some sort of driving stimulus, such as reproduction. However, with imbalances in the body, the animal will have no craving to pursue its natural instincts.
Biochemical Pathway
Researchers have found that the Reward Prediction Errors (RPEs) can be the underlying cause of decreased motivation. When a reward center does not receive a working signal correctly, accurate levels of dopamine cannot be released from the brain. Research shows that with schizophrenia signals of expected outcome is degraded or inaccurate and mechanism for computing RPE is dysfunctional (Cohen & Minor, 2010). The magnitude of avolition depends on the striatal reward prediction errors. Studies have found evidence for reduced positive prediction errors in the striatum, including the caudate and nucleus accumbens (Kumar et al., 2008; Gradin et al., 2011; Robinson et al., 2012). The dysfunctional striatal responses to rewards, proved that changing dopamine levels is important to apathetic actions.
Experiments
In order to fully understand how avolition is related to schizophrenia, different types of experiments were done using neuroimaging to see how the brain responds to different stimuli. A few complications appear like differentiating between general salient responses and actual RPE signals. The responses were tracked in the anterior insula, lateral temporal cortex, and amygdala. By reviewing emotional pictures etc. researchers found that when the patients were shown a positive stimulus the parts of the brain that would normally activate with (+) stimulus, showed less activity than with a (-) stimulus. This concludes that schizophrenics are more motivated by negative consequences, and the typical positive stimuli motivation is lacking. Therefore, researchers found that the majority of the neural function for motivational deficits can be scanned in the fronto- striatal network centered on the ventral and medial regions of the prefrontal cortex. Research has found that schizophrenic patients show abnormal striatal reward anticipation signals. They found that two regions of the brain are incontrol of this: the ventral striatum and ventral PFC (Knutson et al., 2005). In addition, neural signals relating to reward anticipation also correlates with the measures of motivational deficits in not only patients with schizophrenia, but also people with major depressive disorder (Remijnse et al., 2009; Smoski et al. ,2009; Dowd & Barch 2012). However, schizophrenics struggle to accurately represent the value of actions, and therefore, are proven to struggle with lack of motivation since their neural signals function improperly.
Addiction
Drugs can greatly alter how the brain works and lead to addiction. “Drug addiction is a syndrome of dysregulated motivation, evidenced by intense drug craving and compulsive drug-seeking behavior” (Meyer et al., 2015). Different drugs can alter the areas of the brain that controls motivation. The change shifts the motivational systems into “overdrive”. The original brain does not have changes in motivation. The alterations occur after the individual tries the drug. Once the drug is consumed, the individual feels a sense of euphoria and/or lack of anxiety. The positive and negative experiences strengthen the urge to take the drug for a second time (Khantzian 1997; Wise & Bozarth 1987). During the first stages, the individual still has control over their choice since the brain's conformation and chemical balance has not been affected significantly. Currently, researchers lack a complete understanding of the transformation the brain undergoes due to drugs during the transformational phases of controlled to uncontrolled drug intake (Meyer et al., 2015).
Physiological Findings
To understand the motivational process of addiction, rats were administered with different drugs. The results concluded that each drug created different alterations in mesocorticolimbic circuits. The alterations underlie drug-taking behaviors and specifically within basal ganglia circuits (Nestler 2005; Koob & Nestler 1997). Addiction is mediated by multiple motivational processes. For instance, the continuation of drug-taking caused by the negative reinforcement process is a result of removal of a negative drug withdrawal state produced by the drug (Koob & Le Moal 2001). In addition, the hedonic allostasis model emphasizes that drugs produce a reward deficit, which previously pleasurable events now fail to produce the same hedonic effect. Therefore, addiction is maintained by a need to increase the amount of drug used to reach the new hedonic threshold and over anhedonia induced by the drug exposure. Biologically, the new threshold is caused by a decreased activity within dopamine, opioid, and GABA neurotransmitter systems, while anhedonia is mediated by alterations in the functions of the amygdala and corticotropin-releasing hormone systems (Koob & Le Moal 2008; George et al., 2012).
Conclusion
Although there is much yet to be understood regarding the neuroscience of motivation, the pathways of dopamine influence have been attempted to be understood. Theories on the fundamental reward – action systems and any anticipated complications in this neural cascade however have been studied. The foundation of reward-driven motivation (food, sex, social interections) have proven to be based off various factors ranging from hormonal control, learned behavior and external factors. When the motivational pathways of the brain function improperly, studies have found that this can cause issues such as avolition or addiction. However it can be concluded that further studies are still required to fully understand the multiple overlapping systems involved in motivational neuroscience and associated neurological disorders.
References
● Caldwell, H. K., Albers, H. E. (2015). Oxytocin, vasopressin, and the motivational forces that drive social behaviors. In Behavioral neuroscience of motivation (pp. 51-103). Springer, Cham.
● Castel Y. (2014). Simplified diagram of the neurobiological circuits of the lordosis sexual reflex. Retrieved from https://commons.wikimedia.org/wiki/File:Simplified_diagram_of_the_circuits_of_the_lordosis_sexual_reflex.svg
● Cohen, A. S., Minor K.S. (2010). Emotional experience in patients with schizophrenia revisited: metaanalysis of laboratory studies. 36(1):143–150
● Dowd, E. C. & Barch, D. M. (2012). Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PloS one 7(5):e35622
● George, O., Le Moal, M., & Koob, G. F. (2012). Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiology & behavior, 106(1): 58-64.
● Glimcher, P. W. (2011). Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proceedings of the National Academy of Sciences, 108(Supplement 3), 15647-15654.
● Gradin, V. B., Kumar, P., Waiter, G., Ahearn, T., Stickle, C., Milders, M., Reid, I., Hall, J., & Steele, J. D. (2011) Expected value and prediction error abnormalities in depression and schizophrenia. Brain: J Neurol 134(6):1751–1764
● Haber, S. N., & Knutson, B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1): 4-26.
● Hernandez, G., Breton, Y. A., Conover, K., & Shizgal, P. (2010). At what stage of neural processing does cocaine act to boost pursuit of rewards?. PloS one, 5(11).
● Hull E. M., & Rodríguez-Manzo G. (2017) Hormones, Brain and Behaviour, Third Edition.
● Khantzian E. J. (1997) The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev Psychiatry 4(5): 231–244
● Knutson B., Taylor J., Kaufman M., Peterson R. & Glover G. (2005) Distributed neural representation of expected value. J Neurosci 25:4806–4812
● Koob G.F. & Le Moal M., (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2): 97–129
● Kumar, P., Waiter, G., Ahearn, T., Milders, M., Reid, I. & Steele, J.D., (2008) Abnormal temporal difference reward-learning signals in major depression. Brain: J Neurol 131(8):2084–2093
● Kurzban, R., Duckworth, A., Kable, J. W., & Myers, J. (2013). An opportunity cost model of subjective effort and task performance. Behavioral and brain sciences, 36(6): 661-679.
● Lynch, P.J. (2015). Mesolimbic Pathways. Retreived and adapted from https://commons.wikimedia.org/wiki/File:Mesolimbic_pathway.svg
● Magariños, A. M., & Pfaff, D. (2015). Sexual motivation in the female and its opposition by stress. In Behavioral Neuroscience of Motivation (pp. 35-49). Springer, Cham.
● Matsuda, W., Furuta, T., Nakamura, K. C., Hioki, H., Fujiyama, F., Arai, R., & Kaneko, T. (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. Journal of Neuroscience, 29(2): 444-453.
● Meyer, P. J., King, C. P., & Ferrario, C. R. (2015). Motivational processes underlying substance abuse disorder. In Behavioral Neuroscience of Motivation (pp. 473-506). Springer, Cham.
● Mohebi, A., Pettibone, J. R., Hamid, A. A., Wong, J. M. T., Vinson, L. T., Patriarchi, T., Tian, L., Kenedy, R. T., & Berke, J. D. (2019). Dissociable dopamine dynamics for learning and motivation. Nature, 570(7759): 65-70.
● Nestler E. J., (2005). Is there a common molecular pathway for addiction? Nat Neurosci 8(11):1445– 1449
● Newman S. W., (1999). The medial extended amygdala in male reproductive behaviour. A node in the mammalian social behaviour network. Ann NY Acad Sci 877:242–257
● Pfaff D. W., Schwartz-Giblin S (1988). Cellular mechanisms of female reproductive behaviours. In: Knobil E, Neill J (eds) The physiology of reproduction. Raven Press, New York, pp 1487–1568 (Chapter 35)
● Randall, P.A., Lee, C.A., Podurgiel, S.J., Hart, E., Yohn, S.E., Jones, M., Rowland, M., López-Cruz, L., Correa, M. & Salamone, J.D., (2015). Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. International Journal of Neuropsychopharmacology, 18(2):1-11
● Robinson, O. J., Cools, R., Carlisi, C. O., Sahakian, B. J., & Drevets, W. C. (2012). Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. American Journal of Psychiatry, 169(2): 152-159.
● Remijnse, P. L., Nielen, M. M. A., Van Balkom, A. J. L. M., Hendriks, G. J., Hoogendijk, W. J., Uylings, H. B. M., & Veltman, D. J. (2009). Differential frontal–striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive–compulsive disorder. Psychological medicine, 39(9): 1503-1518.
● Salamone J.D., (1991) Behavioral pharmacology of dopamine systems: A new synthesis. In: Willner P, Scheel Kruger J (eds) The mesolimbic dopamine system: from motivation to action. Cambridge University Press: Cambridge, England, pp 598–613
● Salamone, J. D., & Correa, M. (2012). De mystiska motiverande funktionerna av mesolimbic dopamin. Nervcell, 76: 470-485.
● Salamone, J. D., Pardo, M., Yohn, S. E., López-Cruz, L., SanMiguel, N., & Correa, M. (2015). Mesolimbic dopamine and the regulation of motivated behavior. In Behavioral neuroscience of motivation (pp. 231-257). Springer, Cham.
● Steinberg, E. E., Keiflin, R., Boivin, J. R., Witten, I. B., Deisseroth, K., & Janak, P. H. (2013). A causal link between prediction errors, dopamine neurons and learning. Nature neuroscience, 16(7): 966.
● Simpson, E. H., & Balsam, P. D. (2015). The behavioral neuroscience of motivation: an overview of concepts, measures, and translational applications. In Behavioral Neuroscience of Motivation (pp. 1-12). Springer, Cham.
● Shepherd, G. M. (1988). Neurobiology. Oxford University Press.
● Smoski, M. J., Felder, J., Bizzell, J., Green, S. R., Ernst, M., Lynch, T. R., & Dichter, G. S. (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of affective disorders, 118(1-3): 69-78.
● Westbrook, A., & Braver, T. S. (2016). Dopamine does double duty in motivating cognitive effort. Neuron, 89(4): 695-710.
● Woods S.C. (1991). The eating paradox: how we tolerate food. Psychol Rev 98(4):488–505
● Woods S.C., (2009). The control of food intake: behavioural versus molecular perspectives. Cell Metab 9(6):489–49
.● Wise R.A. & Bozarth M.A., (1987). A psychomotor stimulant theory of addiction. Psychol Rev 94 (4):469–492
● Yagishita, S., Hayashi-Takagi, A., Ellis-Davies, G. C., Urakubo, H., Ishii, S., & Kasai, H. (2014). A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science, 345(6204), 1616-1620.
Illustrations
Fig. 1: Dopaminergic pathways of the neural system (redrawn by Lily Molnar) (Lynch, 2015)
Fig. 2: Lordosis stimulation in rats (Castel, 2014)