Rethinking Mucosal Antibody Responses: IgM, IgG, and IgD join IgA
Nicole Lawlor, Sophie Alton Heath, Victoria Stockman
Contents
Abstract
Mucosal Humoral immune responses are usually focused on IgA. In the gut, IgA contributes to the establishment of a mutualistic host–microbiota relationship that is required to maintain homeostasis and prevent disease. This essay discusses how mucosal IgA responses occur in a complex humoral defence network that involves IgM, IgG and IgD. We compare and discuss how IgM, IgG and IgD strengthen communication between mucosal and systemic immune responses .
Introduction
Mucosal IgA have 2 forms Dimeric or Monomeric and Dimeric IgAs control bacteria in the intestinal mucosa. Mucosal IgM is the first line of humoral defence and helps in tissue homeostasis. It is thought that SIgM could cooperate with SIgA to maintain beneficial commensals within gut mucus. Mucosal IgG responds to infection in reproductive and respiratory tract. Mucosal IgD orginates from IgM by class switch recombination.
Mucosal IgA
IgA is the most abundant antibody in mucosal secretions (Cerutti et al, 2011). The gut mucosa is a dynamic interface encompassing an epithelial monolayer that separates the local immune system from diverse communities of commensal bacteria. Commensal bacteria is bacteria that lives in the gut and has a neutral relationship with gut cells (Gutzeit et al, 2014). Gut microbiota allows the intestinal mucosa to fight against pathogens and breakdown food components into vitamins. Commensals stimulate intestinal epithelial cells and increase local immune system (Bäckhed et al, 2005).
IgAs formed by class switch recombination of intestinal B cells by T cell independent and T cell dependent pathways (Fagarasan et al, 2012). In humans we have 2 types of IgA Dimeric intestinal IgA, also called secretory IgA (sIgA) or IgA1 Monomeric serum IgA or IgA2 Whereas mice for example have dimeric IgA is in both gut and serum.
IgA in the gut
Mucosal IgAs interaction with gut bacteria. Microbes stimulate the production of IgA, which controls bacterial translocation and neutralizes bacterial toxins at the intestinal mucosal surface. It is now clear that IgA can function in high-affinity modes for neutralization of toxins and pathogenic microbes, and as a low-affinity system to contain the dense commensal microbiota within the intestinal lumen (Macpherson et al, 2008).
Induction of IgA response
Intestinal IgA production in Peyer’s patches - antigens in the gut initiate mucosal IgA production as gut IgA B cells enter via follicle associated epithelium of Payors Patch and isolated lymphoid follicles in the intestine this causes an immune response by circulation through lymph and bloodstream. They put precursor plasma cells in mucosa which then produce dimeric IgA cells, exported through intestinal epithelium (Macpherson et al, 2008)(Lycke and Bemark, 2012). Multiple germinal centre in Payors patch work synchronously to quality control the gut IgA responses.
How SIgA is released into the gut
M cells (microfold cells) which are in the specialised follicle associated epithelium of payers patch (GALT) capture the luminal SIgA-coated antigens through SIgA receptors. There is interaction with tolerogenic CD4+ T cells which have induce IgA production. (Lindner et al, 2012). In the intestine, monomeric IgA interacts with a small plasma cell derived polypeptide termed joining chain to form IgA dimers that recognize polymeric immunoglobulin receptor (pIgR) on the basolateral surface of mucosal intestinal epithelial cells (Brandtzaeg, 1974). By shuttling IgA dimers across intestinal epithelial cells through a complex process called transcytosis, pIgR facilitates the release of secretory IgA (SIgA) onto the surface of the gut. The resulting, SIgA includes a pIgR-derived polypeptide called secretory component that increases the stability of SIgA in the intestinal lumen and anchors SIgA to mucus (Brandtzaeg and Prydz, 1984).
IgA recognising bacteria
The IgAs recognise bacteria by IG binding FAB segment on the IgA and FC and secretory segments of the molecule. Follicular IgA responses require the activation of CD4+ T cells by antigen-presenting DCs (Gutzeit et al, 2014). IgA1 are released in systemic and mucosal compartments. IgA2 are mostly confined to the gut. In comparison to IgA1 they have a much shorter hinge region which makes them more resistant to degradation by bacterial proteases in the gut. Both IgA1 and IgA2 have an glycosylated FC segment which has the ability to outcompete influenza viruses. They find a FC receptor which has both activating and inhibitory functions in phagocytes.
Commensal bacteria or pathogen?
We are not sure how intestinal IgA discriminates commensals from pathogens and whether specific commensals are needed to optimize homeostatic IgA responses. Further studies could help development of novel oral vaccines, but also contribute to a better understanding of intestinal inflammatory disorders and food allergies (Gutzeit et al, 2014).
Individual IgA repertoires
Studies have shown that intestinal IgAs have low diversity. It is unknow how much somatic recombination and diversification is used in IgAs. A study in mice showed that clones combine to give diverse polyclonal IgA repertoires, with little overlap between individual mice. Expanded clones seed the small intestine but not the large intestine and the diversity increases with age. Each individual IgA repertoire is stable and can be recalled even after plasma cell depletion. This shows functional memory is active, and IgA each repertoire matches the environment and internal stimuli (Lindner et al, 2012).
Immunoglobulins are beneficial to each other, The different types of IGs support each other in ways such as IgA is a ligand for polymeric IG receptor which transport IgA in Mucus epithelial cells and transports pentameric IgM also.
Fetus IgA
Further IgA responses occur in the follicles of mesenteric lymph nodes (MLNs), which develop during fetal life and receive antigens from Peyer Patch via afferent lymphatics (Fagarasan et al, 2010).
Mucosal IgM
IgM is the oldest member of the antibody family according to (Flajnik, 2002), and is the first antibody to appear during B cell ontogeny. Gutzeit et al, 2018 has proven that IgM is the first antibody released in humoral response, it generates first line humoral defence and helps maintain tissue homeostasis (Ehrenstein and Notley, 2010). It is a secretory immunoglobulin at mucosal surfaces and in breast milk.
General properties of mucosal IgM responses.
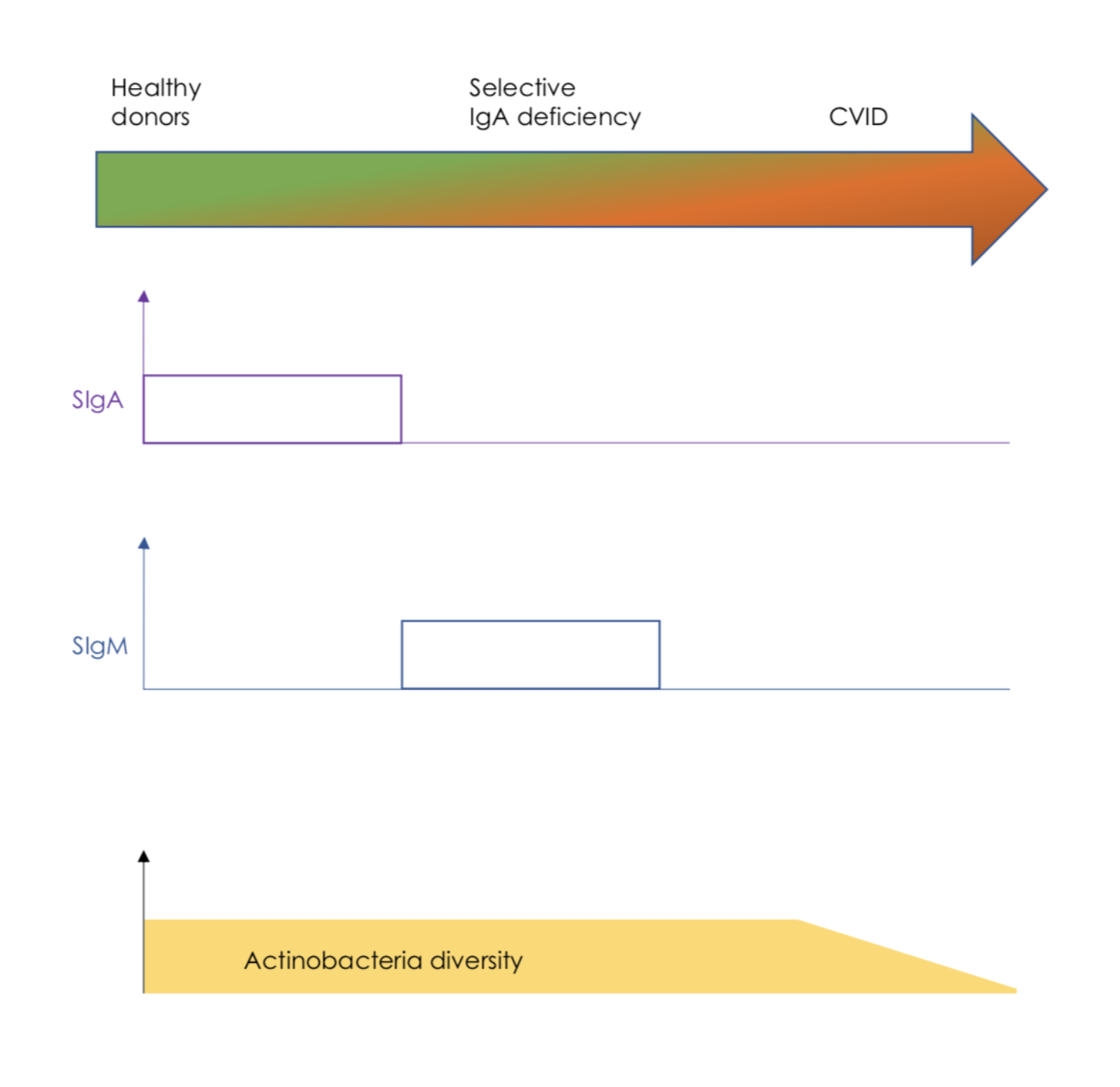
Secreted IgM has multiple immune functions, both immuno-protective and immunoregulatory. It is usually a pentamer but it can be also found as a monomer. Due to its pentameric structure, IgM is a strong agglutinating and strong complement fixing immunoglobulin which is very important for microbial clearance and causing inflammation. (Ehrenstein and Notley, 2010). IgM sends immunoregulatory signals from Fcα/μR receptors and FcμR receptors. Fcα/μR send regulatory signals that help shape humoral immunity, FcμR receptors regulate B cell development (Shibuya and Honda, 2015). There have been additional IgM receptors found in pathogens such as Toxoplasma gondii, these could potentially have a role in evasion from protective IgM responses. As would be expected, individuals with IgM deficiency are prone to recurrent viral, bacterial and protozoal infections, increased IgE mediated allergic and IgG-mediated autoimmune disorders (Gupta, 2017). IgM also enters mucosal secretions as secretory IgM (SIgM). SIgM provides mucosal protection early in life, prior to production of SIgA which is more dominant in later life. Therefore it partially compensates for a lack of SIgA in children and adults with IgA deficiency. It is important to note that SIgM binds to a broader set of gut bacteria than SIgA (Sterlin et al, 2018).
|
Figure 1 In the absence o f IgA, SIgM is able to compensate for a lack of SIgA to maintain actinobacteria diversity. (Fig1)
Cooperation of mucosal IgM and IgA responses .
SIgM coats the gut microbiota from IgA-deficient individuals, and enhances gut bacterial diversity in healthy individuals (Sterlin et al, 2018). It is thought that SIgM could cooperate with SIgA to maintain beneficial commensals within gut mucus, There is a high concentration of SIgM cells in the terminal ileum, they account for approximately 10–20% of total plasma cells (Magri et al, 2017).
It is thought that gut IgM plasma cells need antigen-specific signals for function because they retain surface antigen receptor expression (Pinto et al., 2013). In adults, gut IgM plasma cells are mutated and relate to gut memory B cells. These B cells colonize the intestinal mucosa early in life, mainly in gut-associated lymphoid follicles. According to Magri et al, 2017, Gut memory IgM and IgD B cells undergo IgM-to-IgA recombination in response to T cell independent or T cell Dependent signals, thus in a similar way to IgA response, gut IgM responses may involve IgM diversification from pre-existing memory specificities rather than de novo recruitment of naive B cells (Chen et al, 2020). This allows adults to quickly accommodate SIgM to changes and could lead to the co-release of SIgM and SIgA and maximize mucus retention of beneficial commensals. (Chen et al, 2020)
Mucosal IgG
IgG is more abundant than IgA in reproductive and lower respiratory tracts, and less abundant in the upper respiratory tract, gut mucosa, mammary & lachrymal glands but increases in these areas in response to infection (Brandtzaeg et al, 2001). IgG play roles in mucosal homeostasis and control mucosal bacteria by binding to neonatal fc receptors. In the lumen, IgG works with SIgA and SIgM and IgD. (Rath et al, 2015)
Mucosal IgG subclasses.
IgG responses are pro-inflammatory when working with IgG1, IgG3 and complement activation and Fcγ receptor signalling functions. IgG responses are non-inflammatory when working with IgG2, IgG4, no complement activating and Fcγ receptor signalling functions (Lu et al, 2017). Therefore is gut inflammation is shown by presence of IgG1 and IgG3, whereas a healthy gut is shown by presence of IgG2 and IgG4 (Rengarajan et al., 2019).
Protective functions of mucosal IgG in adults.
IgG specific to commensals are seen in intestinal mucosa and peripheral blood, this suggests that some intestinal IgG-secreting plasma cells come from bone marrow (Fadlallah et al, 2019). It is deducted that there is a specific protective role of IgG in the control of some commensals due to serum IgG showing non-overlapping specificity to the gut microbiota compared to IgA (Fadlallah et al, 2019). IgG can opsonize virulent bacteria, which are then killed by neutrophils (Caballero-Flores et al, 2019). From research on mice we know that protective function of mucosal IgG is not confined to the gut as we see systemic elicitation of IgG response to systemic infections by pathogens like E.coli (Zeng et al., 2016). Thus, Microbiota is not only in the gut lumen, we see that it communicates with extra-intestinal lymphoid organs and provides IgG protection against systemic infections (Chen et al, 2020). Evidence available indicates IgG responses to gut bacteria originate from plasma cells induced by T cell dependant pathways, developing in both gut-associated and systemic lymphoid tissues (Chen et al, 2020).
Protective functions of mucosal IgG in neonates.
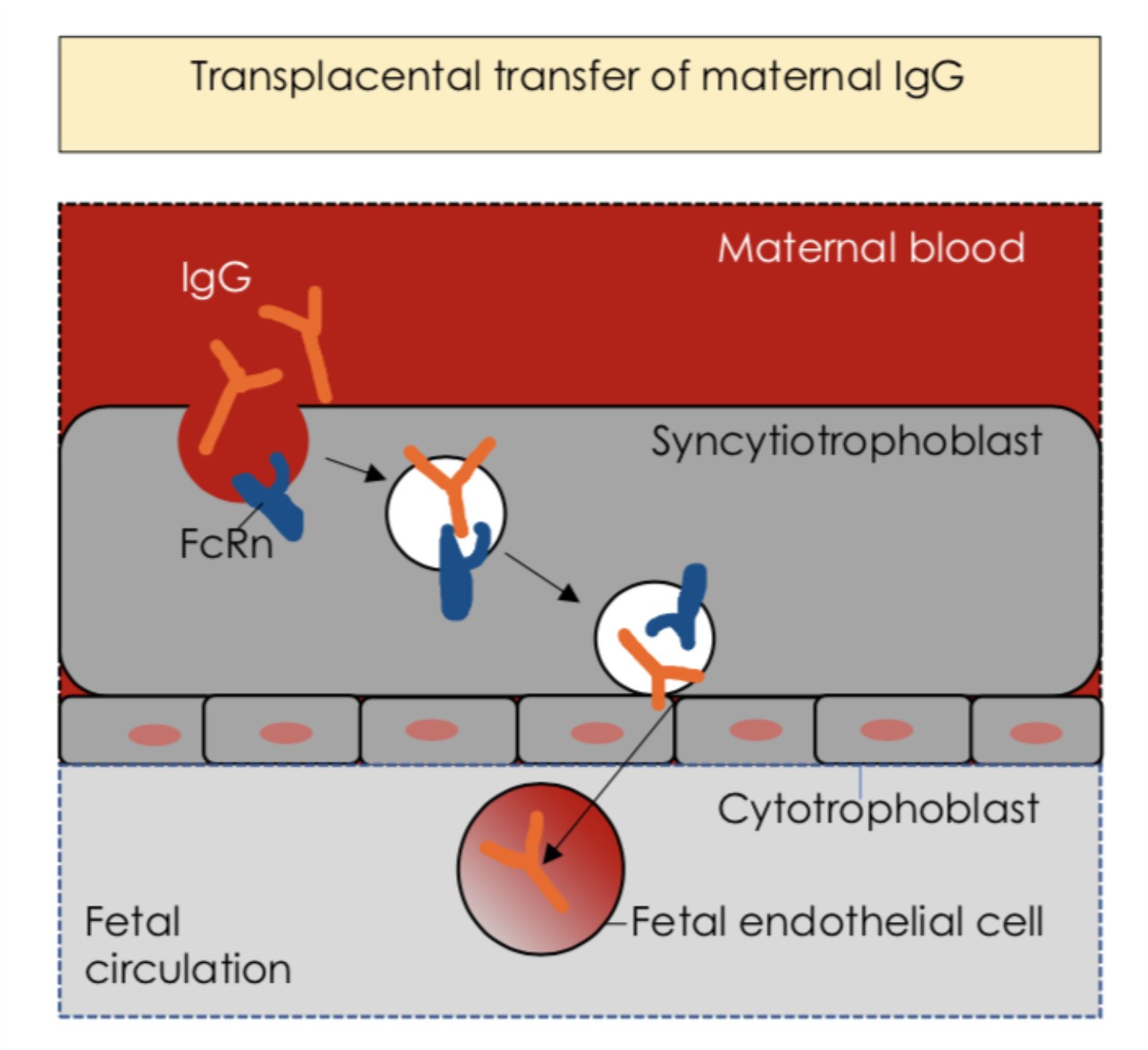
IgG is critical in neonates, it mediates immune adaptation to commensal colonization and aids immune defence against pathogens (Caballero-Flores et al, 2019). This is achieved through many mechanisms, early in life, trans-placental IgG gives protection against common infections, this highlights importance of maternal vaccination. (Rath et al, 2015). These antibodies can be also acquired from maternal milk via intestinal mucosa upon ingestion. Milk-derived SIgA enhances mucosal protection while not being microorganism-specific, but milk-derived IgG needs to be pathogen-specific to protect against mucosal pathogens (Caballero-Flores et al, 2019). Pathogen-specific IgG needs to reach the gut to function, it can be acquired from either the maternal milk or the general circulation via the FcRn (Chen et al, 2020). Milk derived IgG protects against pathogens, promotes intestinal immune maturation, tolerance to microorganisms and enhances allergen protection (Verhasselt et al, 2008). FcRn bidirectionally transports IgG across epithelial cells from intestinal, pulmonary, genital and mammary mucosae, it transports maternal IgG to the foetus across the placenta, providing passive immunity (Rath et al., 2015).
|
Figure 2 IgG in the neonatal gut mucosa. Transplacental transfer of maternal IgG ensures immune protection for the fetus, and the immune maturation of the neonatal gut mucosa. (Fig2)
Mucosal IgD
IgD Secretion IgD is mostly known as a B cell antigen receptor and usually co-expressed with IgM, but it also exists as a secreted antibody. This IgD is released by IgM-IgD+ plasma cells and originates predominantly from mucosal B cells that have undergone IgM-to-IgD CSR (class-switch recombination) as told by (Chen et al, 2020), a mechanism that changes the type of immunoglobin produced by a B cell. This form of class switching from IgM to IgD, occurs in the human upper respiratory mucosa and digestive tracts (Attanasio et al, 2006) (Cerutti et al, 2011). In mice, the IgM-to-IgD CSR happens in nasal-associated lymphoid tissue and submandibular and mesenteric lymph nodes. This class switching is also found in mucosal areas where airborne and oral antigens are detectable. (Chen et al, 2020)
Evolution of IgD
IgD was initially discovered in 1965 as a human myeloma protein (Attanasio et al, 2006). Originally, IgD was thought to be a recently evolved isotype, but new research shows IgD in ancient vertebrates. This suggests that, contrary to initial predictions, IgD has existed for hundreds of million years and has been preserved in evolution from fish to human (Attanasio et al, 2006) (Cerutti et al, 2018). This indicates that IgD has an evolutionary advantage in important immunological functions (Attanasio et al, 2006) (Chen et al, 2020).
The protective role of IgD
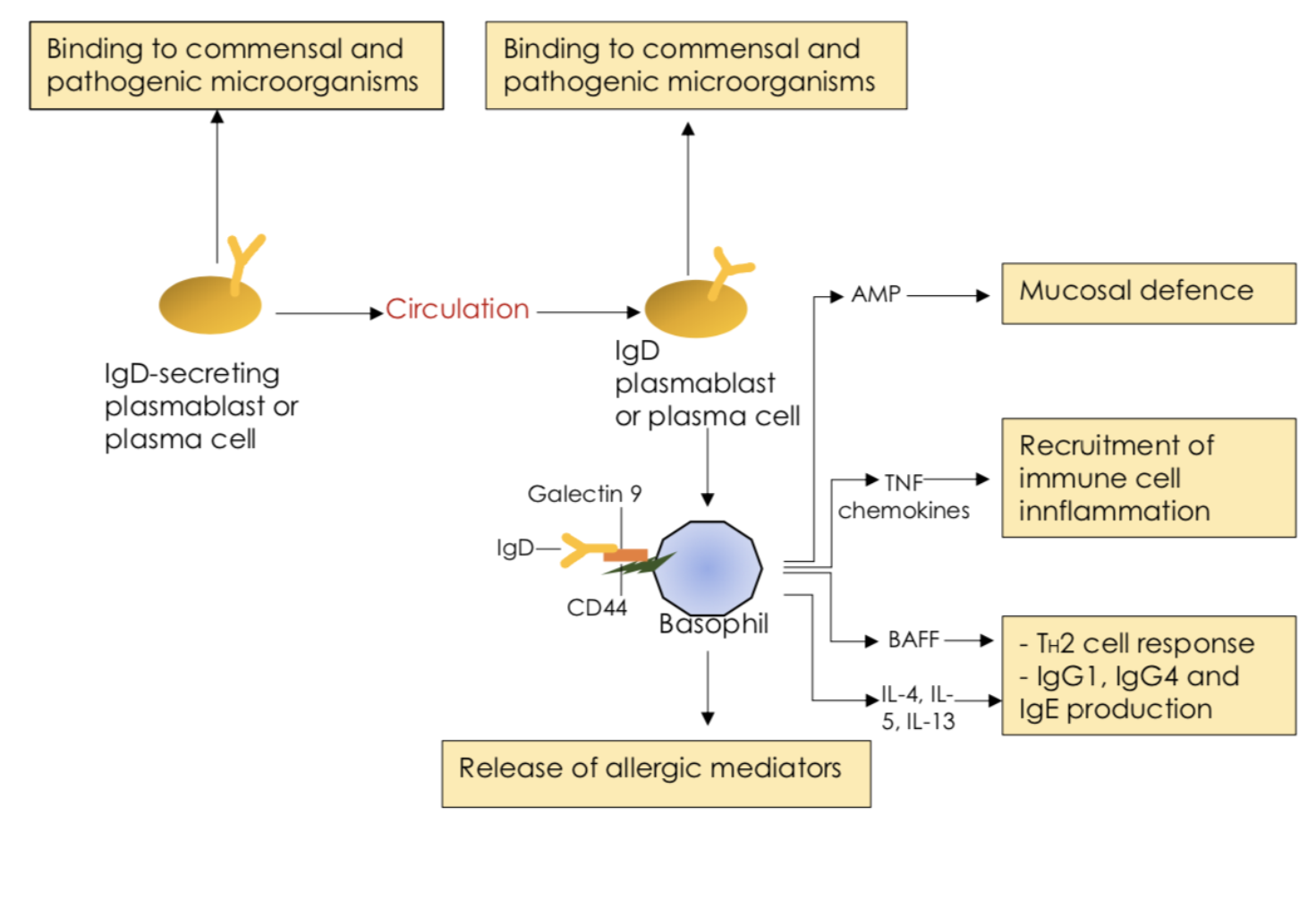
IgD is the least understood of the five antibody classes, not only from an evolutionary perspective, but also from a functional perspective. Its function is poorly understood and is still being researched. The protective role of IgD was suggested by the increased amount of IgD in sera taken from patients with respiratory infections or chronic lung inflammation (Chen et al, 2020). Today, it is known that IgD helps in protection of the respiratory mucosa by binding to pathogenic bacteria. It is believed that IgD contributes to mucosal immunity by neutralizing pathogens and excluding commensals, but also by recruiting basophils as well as other immune cells with antimicrobial and immune-augmenting functions (Cerutti et al, 2011) (Cerutti and Chen, 2011). IgD binds to circulating basophils, monocytes, neutrophils and mucosal mast cells through an unknown receptor which is distinct from IgG, IgA or IgE receptors (Cerutti and Chen, 2011) (Chen et al, 2020). Basophils are cells responsible for inflammatory reactions during immune response, and they also produce compounds that co-ordinate immune responses. Cross-linking of IgD induces the basophil production of immunoactivating cytokines and proinflammatory cytokines. IgD cross-linking also induces basophil release of antimicrobial factors (Cerutti and Chen, 2011). This shows that IgD is responsible for basophils participating directly in antimicrobial immunity.
|
Figure 3 IgD in the aerodigestive mucosa. (Fig3)
Structure and function of mucosal IgD in humans
Human IgD is highly mutated, but it is possible that IgD is diverged from IgM to achieve non-inflammatory immunity. It seems like IgD molecules lack the structural requirements for several effector functions, such as complement activation. Instead of activating complement, IgD is able to recruit lectins, like galectin 9, which is a lectin with both activating and inhibitory immune functions (Chen et al, 2020).
IgD is a heavy chain immunoglobin. Heavy chain immunoglobulins have a hinge region for added flexibility. The hinge region in IgD is long and it is uniquely encoded by two exons. The structure and length of the hinge region gives IgD a T-shape rather than the typical Y-shape of other antibody isotypes (Cerutti and Chen, 2011). The T-shape gives IgD more binding flexibility, which helps IgD to bind low-density epitopes and polyvalent antigens, such as immune complexes (Chen et al, 2020) (Cerutti and Chen, 2011).
IgD in secreted form is also characterized by low proline content and high carbohydrate content. It also has a high susceptibility to proteolysis, cleavage by bacterial proteases, because of its long hinge region (Howard et al, 1975). These properties suggest a unique structure of IgD within the structural pattern of general immunoglobulins with two heavy and two light polypeptide chains. The human IgD chain has up to seven O-linked glycans. The immune-augmenting properties of IgD are linked to the O-glycans which are able to bind lectins, including galectins (Chen et al, 2020) (Cerutti and Chen, 2011).
Species-specific properties of mucosal IgA, IgM, IgG and IgD
IgA
In humans, mucosal IgA is divided into two subclasses, IgA1 and IgA2. This division does not exist in mice (Cerutti et al, 2018). Human IgA1 binds to Fcα receptor I. FcαRI is found on the surface of neutrophils, eosinophils, monocytes, some macrophages like Kupffer cells, and some dendritic cells. Mouse IgA lacks an FcαRI homologue and is unable to bind to the receptor (Chen et al, 2020).
IgM
The concentrations of free secretory IgM (SIgM) and SIgM-coated bacteria in mice is small compared to the concentrations in human, and it is not worth considering (Chen et al, 2020). This is the result of the gut lamina propria in mice does not have gut plasma cells releasing IgM. In human gut lamina propria, on the other hand, 20% of the total plasma cells secretes IgM (Albero- Gonzáles et al, 2017).
IgD
There are structural differences in the hinge region of IgD that contribute to different functions of IgD in humans and mice (Cerutti and Chen 2011). A functional antibody is constructed by gene products; variable (V) region, joining (J) region, constant (C) region, and diversity (D) gene in heavy chains. Human IgD is highly mutated and IgD includes up to 80V(D)J gene mutations and has a clear center derivation (Cerutti et al, 2018). Mouse IgD is poorly mutated compared to human IgD (Chen et al, 2020).
IgG
IgG is divided into four subclasses. The subclasses are based on differences in the heavy chain constant regions. Structural differences in the Fc regions and the hinge regions of the different subclasses assign different functions to the different subclasses (Knutsen, 2019). The gut IgG1 responses detected in mice and the human gut IgG4 and IgG2 responses, seems to be equivalent (Chen et al, 2020)
Conclusion
Recent advances in scientific research have opened our eyes and found new details to the mechanisms surrounding mucosal IgA responses, and has further explained how these responses control commensal and pathogenic bacteria. Additional studies have started revealing the impact of IgM and IgD on mucosal homeostasis and have showed how IgG cooperates with IgA to optimize homeostasis, tolerance and immunity. This new knowledge will could potentially lead way to the development of new antibody-based therapies against infectious, inflammatory and allergic disorders.
References
Albero-Gonzáles, R.; Bascones, S.; Cerutti, A.; Comerma, L.; Grasset, E. K.; Gutzeit, C.; Iglesias, M.; Lligé, D.; Magri, G.; Márquez, L.; Mercade, E.; Mehandru, S.; Pybus, M.; Ramanujam, M.; Segura-Garzón, D.; Serrano, S.; Sintes, J.; Uzzan, M.; van Zelm, M. C.; Vazquez, I.; Yeste, A. (2017): Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals, Cell Press: Immunity, 47:(1), pp. 118-134, DOI: 10.1016/j.immuni.2017.06.013
Attanasio, R.;Richardson, J.; Rogers, K.; Scinicariello, F.(2006): Molecular characterization of immunoglobulin D in mammals: immunoglobulin heavy constant delta genes in dogs, chimpanzees and four old world monkey species. Immunology, 118:(1), pp.88-100, DOI: 10.1111/j.1365-2567.2006.02345.x
Backhed, F. (2005): Host-Bacterial Mutualism in the Human Intestine. Science, 307:(5717), pp.1915-1920. DOI: 10.1126/science.1104816
Brandtzaeg, P. (1974): Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature, 252:(5482), pp.418-420.DOI: 10.1038/252418a0
Brandtzaeg, P.; Baekkevold, E. Morton, H.(2001): From B to A the mucosal way. Nature Immunology, 2:(12), pp.1093-1094. DOI: 10.1038/ni1201-1093
Brandtzaeg, P.; Prydz, H.(1984): Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature, 311:(5981), pp.71-73.DOI: 10.1038/311071a0
Caballero-Flores, G.; Sakamoto, K.; Zeng, M.; Wang, Y.; Hakim, J.; Matus-Acuña, V.; Inohara, N.; Núñez, G. (2019): Maternal Immunization Confers Protection to the Offspring against an Attaching and Effacing Pathogen through Delivery of IgG in Breast Milk. Cell Host & Microbe, 25:(2), pp.313-323.e4. DOI:10.1016/j.chom.2018.12.015
Cerutti, A.; Chen, K.; Chorny, A.(2011): Immunoglobulin Responses at the Mucosal Interface. Annual Review of Immunology, 29:(1), pp.273-293, DOI: 10.1146/annurev-immunol-031210-101317
Cerutti, A.;Chen, K.(2011): The function and regulation of immunoglobulin D. Current Opinion in Immunology, 23:(3), pp.345-352, DOI: 10.1016/j.coi.2011.01.006
Cerutti, A.; Chen, K.; Gutzeit, C.(2018): The enigmatic function of IgD: some answers at last, European Journal of Immunology, 48:(7), pp.1101-1113, DOI: 10.1002/eji.201646547
Chen, K.;Magri, G.; Grasset, E.; Cerutti, A.(2020): Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nature Reviews Immunology,. DOI:10.1038/s41577-019-0261-1
Ehrenstein, M.; Notley, C.(2010): The importance of natural IgM: scavenger, protector and regulator. Nature Reviews Immunology, 10:(11), pp.778-786. DOI: 10.1038/nri2849
Fadlallah, J.; Sterlin, D.; Fieschi, C.; Parizot, C.; Dorgham, K.; El Kafsi, H.; Autaa, G.; Ghillani-Dalbin, P.; Juste, C.; Lepage, P.; Malphettes, M.; Galicier, L.; Boutboul, D.; Clément, K.; André, S.; Marquet, F.; Tresallet, C.; Mathian, A.; Miyara, M.; Oksenhendler, E.; Amoura, Z.; Yssel, H.; Larsen, M.; Gorochov, G.(2019): Synergistic convergence of microbiota-specific systemic IgG and secretory IgA. Journal of Allergy and Clinical Immunology, 143:(4), pp.1575-1585.e4. DOI: 10.1016/j.jaci.2018.09.036
Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. (2010): Adaptive Immune Regulation in the Gut: T Cell–Dependent and T Cell–Independent IgA Synthesis. Annual Review of Immunology, 28:(1), pp.243-273. DOI: 10.1146/annurev-immunol-030409-101314
Flajnik, M.(2002): Comparative analyses of immunoglobulin genes: surprises and portents. Nature Reviews Immunology, 2:(9), pp.688-698 DOI: 10.1038/nri889
Gupta, S.; Gupta, A.(2017): Selective IgM Deficiency—An Underestimated Primary Immunodeficiency. Frontiers in Immunology, 8. DOI: 10.3389/fimmu.2017.01056
Gutzeit, C.; Chen, K.; Cerutti, A.(2018): The enigmatic function of IgD: some answers at last. European Journal of Immunology, 48:(7), pp.1101-1113. DOI:10.1002/eji.201646547
Gutzeit, C.; Magri, G.; Cerutti, A. (2014): Intestinal IgA production and its role in host-microbe interaction. Immunological Reviews, 260:(1), pp.76-85. DOI: 10.1111/imr.12189
Howard, A.; Johnson, P.; Scopes, P. (1975): The conformation of human immunoglobulin D. FEBS Letters, 49:(3), pp.310-313. DOI: 10.1016/0014-5793(75)80773-3
Knutsen, A. P. (2019): IgG subclasses: Physical properties, genetics and biological functions. UpToDate[Accessed 19 April 2020]
Lindner, C.; Wahl, B.; Föhse, L.; Suerbaum, S.; Macpherson, A.; Prinz, I.; Pabst, O.(2012): Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. The Journal of Experimental Medicine, 209:(2), pp.365-377. DOI: 10.1084/jem.20111980
Lu, L.; Suscovich, T.; Fortune, S.; Alter, G.(2017): Beyond binding: antibody effector functions in infectious diseases. Nature Reviews Immunology, 18:(1), pp.46-61. DOI: 10.1038/nri.2017.106
Lycke, N.; Bemark, M.(2012): The role of Peyer’s patches in synchronizing gut IgA responses. Frontiers in Immunology, 3. DOI: 10.3389/fimmu.2012.00329
Magri, G.; Comerma, L.; Pybus, M.; Sintes, J.; Lligé, D.; Segura-Garzón, D.; Bascones, S.; Yeste, A.; Grasset, E.; Gutzeit, C.; Uzzan, M.; Ramanujam, M.; van Zelm, M.; Albero-González, R.; Vazquez, I.; Iglesias, M.; Serrano, S.;Márquez, L.; Mercade, E.; Mehandru, S. Cerutti, A. (2017): Human Secretory IgM Emerges from Plasma Cells Clonally Related to Gut Memory B Cells and Targets Highly Diverse Commensals. Immunity, 47:(1), pp.118-134.e8. DOI: 10.1016/j.immuni.2017.06.013
Macpherson, A.; McCoy, K.; Johansen, F.; Brandtzaeg, P. (2007): The immune geography of IgA induction and function. Mucosal Immunology, 1:(1), pp.11-22. DOI: 10.1038/mi.2007.6.
Pinto, D.; Montani, E.; Bolli, M.; Garavaglia, G.; Sallusto, F.; Lanzavecchia, A.; Jarrossay, D.(2013): A functional BCR in human IgA and IgM plasma cells. Blood, 121:(20), pp.4110-4114. DOI: 10.1182/blood-2012-09-459289
Rath, T.; Baker, K.; Pyzik, M.; Blumberg, R. (2015): Regulation of Immune Responses by the Neonatal Fc Receptor and Its Therapeutic Implications. Frontiers in Immunology, 5. DOI: 10.3389/fimmu.2014.00664
Rengarajan, S.; Vivio, E.; Parkes, M.; Peterson, D.; Roberson, E.; Newberry, R.; Ciorba, M.; Hsieh, C., (2019): Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes, pp.1-16. DOI: 10.1080/19490976.2019.1626683
Shibuya, A.; Honda, S. (2015): Immune regulation by Fcα/μ receptor (CD351) on marginal zone B cells and follicular dendritic cells. Immunological Reviews, 268(1), pp.288-295. DOI: 10.1111/imr.12345
Sterlin, D.; Fieschi, C.; Malphettes, M.; Larsen, M.; Gorochov, G.; Fadlallah, J. (2018): Immune/microbial interface perturbation in human IgA deficiency. Gut Microbes, 10:(3), pp.429-433. DOI: 10.1080/19490976.2018.1546520
Verhasselt, V.; Milcent, V.; Cazareth, J.; Kanda, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V.(2008): Breast milk–mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nature Medicine, 14:(2), pp.170-175. DOI: 10.1038/nm1718
Zeng, M.; Cisalpino, D.; Varadarajan, S.; Hellman, J.; Warren, H.; Cascalho, M.; Inohara, N.; Núñez, G.(2016): Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity, 44:(3), pp.647-658. DOI: 10.1016/j.immuni.2016.02.006
Figure 1: Schematic drawing by us, adapted from Sterlin et al., 2018.
Figure 2: Schematic drawing by us, adapted from Chen et al., 2020.
Figure 3: Schematic drawing by us, adapted from Chen et al., 2020.