|
Size: 20329
Comment:
|
Size: 20734
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 37: | Line 37: |
| The neuroendocrine system in the process in which the hypothalamus maintains homeostasis. The endocrine system and the nervous system are interlinked and the two combined control the each other. Endocrine system consists of hormones produced by various endocrine glands(hypothalamus and pituitary) around the body which are involved in regulating metabolism, growth, tissue function and sexual reproduction. For these hormone to be effective there must be an interface between the hormones and nervous system so that they can act on organs. The hypothalamus links endocrine and nervous system through the pituitary gland. (Norman T.Adler) | The neuroendocrine system is the primary mechanism by which the organism maintains homestasis in response to internal and environmental stimuli. The main way this is achieved is through mediation of the secretory behaviour of the hypothalamus, pituitary gland and the neural output of the autonomic nervous system. |
| Line 39: | Line 39: |
| Three pathways are important when considering the neuroendocrine system: | The endocrine system is comprised of all tissues and cells that secrete hormones. These hormones are responsible for modulating a wide array of bodily processes related to digestion, metabolism, growth & development, electrolyte and fluid balance and sexual reproduction. |
| Line 41: | Line 41: |
| HPA: Hypothalamus- Pituitary-Adrenal axis | The nervous system interacts with the endocrine system by accumulating and assimilating stimuli and passing on said stimuli to the endocrine system to adapt it's secretory behaviour appropriately. |
| Line 43: | Line 43: |
| HPG: Hypo-pituitary-gonad axis | The mian nexus by which these two systems interface is the hypothalamus and pituitary gland. (Norman T.Adler) |
| Line 45: | Line 45: |
| HPT: Hypothalamus-pituitary-thyroid | The three pathways of most importance in the neuroendocrine system are as follows: |
| Line 47: | Line 47: |
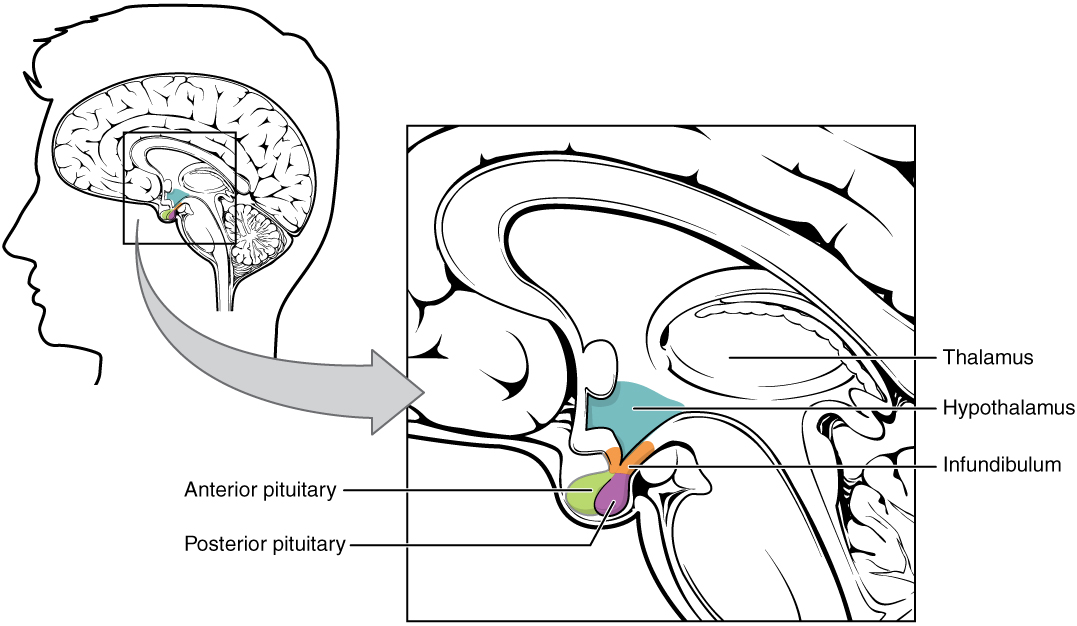
| The hypophysis is separated in two parts neurohypophysis (posterior lobe) and adenohypophysis (anterior lobe).The neurohypophysis is an extension of the hypothalamus. Oxytocin and vasopressin are two hormones produced in hypothalamus but stored and secreted in neurohypophysis. Oxytocin stimulates the uterus especially during late pregnancy and vasopressin regulates loss of water. | - Hypothalamus-pituitary-adrenal axis (HPA). - Hypothalamus-pituitary-gonad axis (HPG). - Hypothalamus-pituitary-thyroid axis (HPT). The hypophysis is separated in two parts neurohypophysis (posterior lobe) and adenohypophysis (anterior lobe). The neurohypophysis is an extension of the hypothalamus. While this gland is responsible for the synthesis and secretion of a large percentage of the hormone reservoirs in the body, some are synthesised in the hypothalamus and secreted by the neurohypophsis, e.g. oxytocin and vasopressin are two hormones produced in hypothalamus but stored and secreted in neurohypophysis. |
| Line 56: | Line 62: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:hypo1.jpg|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''Hypothalamic-pituitary axis'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:hypo1.jpg|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''Hypothalamic-pituitary axis'' || |
| Line 92: | Line 98: |
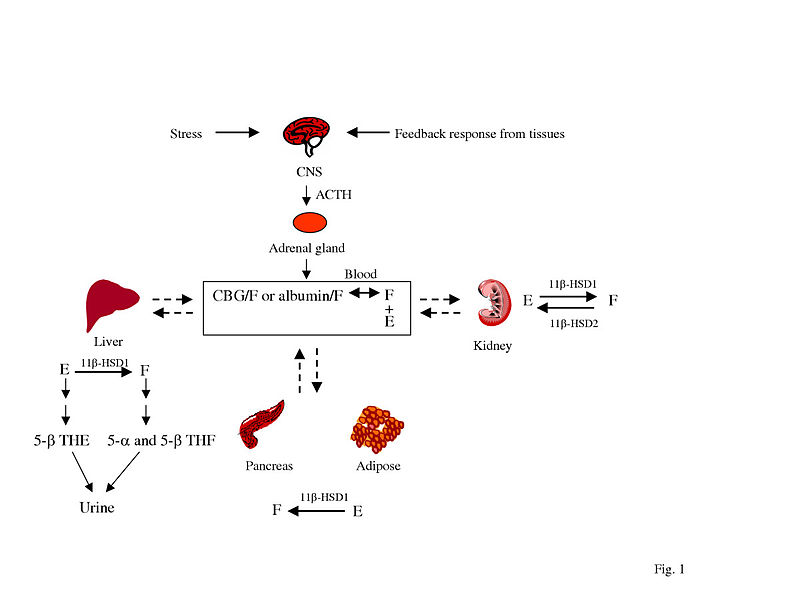
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:ACTH.jpg|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''ACTH cortisol feedback loop'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:ACTH.jpg|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''ACTH cortisol feedback loop'' || |
The effects of overtraining on the neuroendocrine system.
Contents
Introduction.
In the following text we will attempt to clarify what exactly is meant by the term overtraining (OT), also known as overtraining syndrome (OTS), including differentiating it from the similar condition of overreaching. While outlining the pathophysiology we will explore what is meant by the term stress as it plays a vital role in the onset of overtraining.
We will then describe how the neuroendocrine system functions under normal physiological conditions.
Once the parameters of overtraining and the normal functioning of the neuroendocrine system have been defined we will explore how the two interact with each other, paying particular attention to how the multiple proposed hypotheses for the mechanisms of action compound their respective symptoms.
Finally, we will indicate methods by which the severity of overtraining can be mitigated and ideally, completely avoided.
Overtraining
Overtraining is considered “an extreme condition of maladapted physiology” (Kreher, 2016).
It is the net result of demanding levels of mechanical work of the body at rates above which it is physiologically capable of carrying out or withstanding. The resulting stress or physical trauma is a consequence of the rate of work exceeding the rate at which the body can repair the damage created by said work. When the balance between training and recovery is disproportionately weighted towards training, minor fatigue and performance reduction ensue (Halson, 2004).
Overtraining is not simply excessive work independent performance decline, but when the former causes the latter. (Smith LL, 2000). Symptoms negatively impact a wide range of bodily systems over a prolonged period of time including hormonal, immunological, neurological and psychological systems (Meeusen R et al., 2013). Overtraining greatly impacts the hypothalamic-pituitary-adrenal and hypothalamic-adrenal-gonadal endocrine axes and the sympathetic-adrenal-medullary neurological axis (Fry RW et al., 1991).
Overreaching can be considered a mild form of overtraining and a prerequisite, in combination with other factors, to development of acute overtraining syndrome. It is the result of engaging in training volume or intensity that result in temporary performance decline. If given adequate rest and isolated from excessive stress, overreaching results in performance improvements due to super-compensatory physiological adaptations. It can thus be thought of as overtraining in the short term. If the volume and intensity of training is carried out at levels that would qualify as overreaching but are practiced over longer periods of time with inadequate rest and exposure to stressors, overtraining syndrome (OTS) results (Kreher, 2016).
It is important to underscore the fact that prolonged periods of excessive physical exertion coupled with suboptimal recovery can only develop into OTS when both happen concurrently with stress. This stress can be that caused by the overtraining itself, or some other stressor unrelated to training itself, such as emotional or environmental stress (Carfagno, 2014).
Stress.
Stress is defined as “a real or interpreted threat to the physiological or psychological integrity (i.e., homeostasis)” (Anthony C Hackney, 2010). The endocrine system responds to overtraining induced stress through mediation of the activity of the hypothalamus and thus, the pituitary gland and autonomic
nervous system The pituitary gland and autonomic nervous system are responsible for controlling the activity of a wide range of critical body processes. Altering their behaviour can elicit acute changes in multiple organ systems resulting in pronounced, rapid responses tailored specifically to alleviating stress. (Anthony C Hackney, 2010).
Before discussing how stress impacts the endocrine system it is important to first clarify how it perceives stress in the external environment. The primary pathway by which the endocrine system is mobilised in response to stress is through visual and auditory stimuli. When confronted with an environmental stressor, such as an abnormally loud noise, potential combative actor or supernormal heat the sensory input is channelled to the amygdala, the seat of emotional processing in the brain. If the amygdala perceives the stimulus as a potential danger, it relays signals to the hypothalamus which in turn alters the activity of the autonomic nervous system and the endocrine system, primarily through the altercation of the secretion of hormones. The amygdala can also trigger a stress response based solely on unpleasant emotional triggers, e.g. fearful faces, fear inducing images and fear conditioned cues (Kerry J. Ressler, 2011).
To the endocrine system, stress and danger are synonymous. Thus, when stressed, it is imperative that the endocrine system activate pathways that facilitate rapid response to mitigate perceived danger. Real or anticipated imminant danger can rapidly alter physiology, for example the sympatho-adrenomedullary system can quickly elevate heart rate and blood pressure (Yvonne et al., 2014).
Along with non-endocrine responses, such as the involuntary evacuating of the bowels, there are noted and acute responses by the endocrine system (Olaru et al., 2016).The primary shift in homeostatic equilibrium is in that of energy demand. To protect against danger, the main instinctive responses are the famed “fight or flight” responses. These require a substantial increase in the energy demands of both the muscular and nervous systems. This allows the organism to increase it’s capability to carry out mechanical work in defensively attacking or fleeing the stressor. It also facilitates an increase in the rate at which the nervous system can process sensory input. This response is of vital importance in fast paced, life-and-death situations in which split seconds can be the difference between surviving or not (Jeffrey and Underhill, 1998).
How neuroendocrine system functions under normal physiological conditions.
The neuroendocrine system is the primary mechanism by which the organism maintains homestasis in response to internal and environmental stimuli. The main way this is achieved is through mediation of the secretory behaviour of the hypothalamus, pituitary gland and the neural output of the autonomic nervous system.
The endocrine system is comprised of all tissues and cells that secrete hormones. These hormones are responsible for modulating a wide array of bodily processes related to digestion, metabolism, growth & development, electrolyte and fluid balance and sexual reproduction.
The nervous system interacts with the endocrine system by accumulating and assimilating stimuli and passing on said stimuli to the endocrine system to adapt it's secretory behaviour appropriately.
The mian nexus by which these two systems interface is the hypothalamus and pituitary gland. (Norman T.Adler)
The three pathways of most importance in the neuroendocrine system are as follows:
- Hypothalamus-pituitary-adrenal axis (HPA).
- Hypothalamus-pituitary-gonad axis (HPG).
- Hypothalamus-pituitary-thyroid axis (HPT).
The hypophysis is separated in two parts neurohypophysis (posterior lobe) and adenohypophysis (anterior lobe). The neurohypophysis is an extension of the hypothalamus. While this gland is responsible for the synthesis and secretion of a large percentage of the hormone reservoirs in the body, some are synthesised in the hypothalamus and secreted by the neurohypophsis, e.g. oxytocin and vasopressin are two hormones produced in hypothalamus but stored and secreted in neurohypophysis.
Neurohormones differ from true hormones as they are secreted by neurosecretory cells. Hormones of parvocellular area go to adenohypophysis and consist of supraoptic nucleus and paraventricular nucleus, and contains oxytocin and ADH. Hypothalamic neurosecretory cells are stimulated by
neurotransmitters (e.g. dopamine, serotonin) to release their hormones to convert neural information to hormonal output. Parvocellular neurosecretion goes down axon and reaches portal circulation and further reaches anterior lobe of pituitary gland. It releases hormones into vascular system. Magnocellular go to the neurohypophysis and consist of ventromedial, ventrodorsal and infundibular nucleus. ( George Fink, Donald W. Pfaff, Jon E. Levine)
Growth hormone: the secretion of growth hormone is regulated through neuroendocrine control system through use of hypophysiotropic hormones, GnRH (GH releasing hormone) and somatostatin. (Eugenio E. Müller, Vittorio Locatelli and Daniela Cocchi)
There are 9 hypothalamic hypophysiotropic hormones which regulate release of adenohypophysis hormones. The hypothalamic hormones are secreted from neuroendocrine cells in a number of different hypothalamic nuclei. ( Richard E. Brown)
|
Impact of overtraining on the neuroendocrine system.
Although OTS is a relatively new field of study, there are several promising yet unproven theorised mechanisms of action. They are as follows:
- Cytokine Hypothesis
- Hypothalamic Hypothesis
- Glycogen Hypothesis
- BCCA hypothesis.
The cytokine hypothesis.
This rests on the assumption that the local inflammatory response stimulated by training induced micro trauma becomes chronic due to poor recovery and stress. This leads to an excessive immune response and associated pathophysiology. Certain cytokines act upon the hypothalamus, leading to a decrease in hunger. This is a well documented symptom of elevated cytokine levels. Micro infusion with cytokines can predictably lower subsequent caloric intake by reducing both meal size and duration in a clinical setting “IL-1 beta and IFN act directly and specifically on the glucose-sensitive neurons in the ventromedial hypothalamic nucleus (a ‘satiety’ site) and the lateral hypothalamic area (a ‘hunger’ site)” (Kreher 2012). This reduced appetite and consequent drop in caloric intake depletes the glycogen stores of muscle tissue. TNF-alpha also down regulates protein synthesis and thus GLUT-4 transporter availability. Decreased GLUT-4 transporters compound the endocrine mediated glycogen deficit and lead to the underperformance of muscle tissue.
Hypothalamic hypothesis.
Overtraining can impair the normal regulatory mechanisms that ensure hypothalamic homeostasis. The HPA and HPG axes are the most severely impacted by overtraining. Stark deviations from normal physiological levels can be seen in the secretion and plasma concentrations of the following hormones:
* Cortisol
* Adrenocorticotrophic hormone (ACTH).
* Testosterone (and consequentially oestrogen).
(Armstrong, 2002).
Cortisol and adrenocorticotrophic hormone.
Depletion of serum cortisol is indicative of adrenal fatigue, resultant of overstimulation due to stress. Low cortisol levels properly account for many of the parasympathetic manifestations of OTS including fatigue, depression, bradycardia and a loss of motivation. (Carfagno, 2014. Table 1).
Prolonged training causes sustained secretion of ACTH. Over time, in an effort to achieve homeostasis, there is a downregulation of autonomic responsiveness and sensitivity. The pituitary compensates for this by increasing secretion of ACTH. The increased secretion of ACTH can temporarily mask and obscure the underlying pathophysiology. This can not be sustained indefinitely and without sufficient rest the overworked adrenal glands will not be able to respond to the elevated ACTH levels. At this stage, the organism will display symptoms typical of Addison’s disease: fatigue, lack of ambition, weight loss.
This hypothesis adequately combines many of the mechanisms of action and symptoms of OTS.
|
Glycogen hypothesis.
Local glycogen stores in muscle tissue are the primary energy source for carrying out work. Low glycogen increases oxidation and decreased levels of BCAA’s. BCAA’s are required for CNS neurotransmitter synthesis. The lack of available energy to carry out work and lowered levels of precursors required for optimal neural signalling account for many of the underperformance and fatigue related symptoms associated with OTS. (Meeusen, 2013). Glycogen depletion can occur directly via excessive demands being placed on the stores in situ (Jensen, 2011). This can also be exacerbated by a delay in the replenishment of it’s synthesis due to the cytokine mediated decrease in appetite and downregulation of GLUT-4
transporters. Glycogen depletion is not a perfect hypothesis. OTS can occur parallel with the maintenance of sufficient glycogen stores for the duration of training. This highlights the obscure and multifaceted nature of the development of OTS.
Epinephrine (adrenaline).
As discussed at length, stress in integral to development of overtraining. Epinephrine is of paramount importance in relation to the endocrine system’s response to stress. It induces a plethora of physiological changes, all of which aid in combating stressful situations, namely increased;
-Cardiac output.
-Pupil dilation.
-Blood flow to muscles.
-Blood sugar.)
The increase cardiac output accounts for the typical rise in heart rate and blood pressure associated with overtraining (Hynyen et al., 2009).
Methods to mitigate the negative outcomes of overtraining.
Adequate recovery.
In the case of overtraining neuroendocrine system it reflects the exhaustion stage of Selye’s General Adaption Syndrome (the way the body resonds to stress). In case of excessive musculoskeletal exercise which results in tissue damage, system inflammation develops and becomes excessive. Inflammatory cytokines act on HPA axis especially paraventricular nucleus where corticotropin releasing hormone production occurs which influences ACTH secretion. This will have negative effect on mood and immune function and will intensify, compromising their health. To mitigate this, the person must allocate more time to rest-recovery to enable neuroendocrine system to respond. (Hackney, 2006)
Stress management.
Other ways to prevent overtraining of neuroendocrine system is to monitor all aspects of your life. Record your trainings and how they went, record sleep quality, fatigue, stress, muscle soreness, any illnesses and causes of stress. Through this athletes can quicker identify anything in their lifestyle that may lead to Overtraining syndrome (OTS). (Meeusen, 2006 )
Eating high levels of carbohydrates and amino acids can help prevent overtraining. After hard training, carbohydrate depletion is linked with fatigue therefore carbohydrate supplementation can reverse the symptoms. When the daily carbohydrate intake was increased, the drops in performance were much smaller. Carbohydrate depletion causes increased stress response (cortisol, glucagon and catecholamine increased). To prevent overtraining of neuroendocrine system, an athlete could increase their carbohydrate intake more regularly (before and after they training) to counteract the effects of carbohydrate depletion i.e increased stress which develops the rick of developing chronic fatigue or overtraining syndrome(OTS).
High levels of amino acids also prevent overtraining of neuroendocrine system. After exercise glutamine levels generally fall therefore abnormally low levels of plasma glutamine is a characteristic of overtrained athletes. Plasma glutamine levels increase temporarily after a meal. Prolonged recovery after training may allow the glutamine levels to rise again. Plasma glutamine levels are a good indicator of overtraining system so to prevent this one must supplement amino acids and have a longer recovery time. ). (Romain Meeusen, Martine Duclos, France Carl Foster, Andrew Fry, Michael Gleeson, David Nieman, John Raglin, Gerard Rietjens, Ju¨rgen Steinacker, Axel Urhausen)
Hyperthermic conditioning.
Finally hyperthermic conditioning may have positive effects on the endocrine system. Patients exposed to dry heat in a sauna for a few days saw levels of ACTH and cortisol levels drop while growth hormone and prolactin increased in males. As a result the effects of hyperthermic condtions reduced stress on neuroendocrine system. (Leppäluoto, 1986)
Conclusion.
Overtraining is a complex and nuanced syndrome that has yet to be fully understood by the medical community. The wide array of biological systems on which it has a negative impact make simple diagnostic methods with narrow parameters difficult or impossible to design. Added to this, the fact that stress is essential to the development of overtraining and it too is an eclectic condition compounds the difficulty in pinpointing direct cause and effect.
Overtraining is a debilitating syndrome that can have drastic negative outcomes on both athletic performance and general ability to withstand the routine stresses of everyday life. It should be at the forefront of athlete's and trainer’s minds so as to ensure both optimal performance in the short term and longevity of athletic career.
Although it seems counterintuitive to the layperson, greater intensity, duration and frequency of training does not have a linear relationship with improved athletic outcomes. There should be a close monitoring for of the onset of symptoms of OTS continuously throughout the entire training regimen.
Sufficient periods of rest and recovery should be predetermined and built in to any efficient training plan.
Tools for stress management and avoidance should be adopted by any athlete with similar enthusiasm and dedication to that applied to the mastery of biomechanical training techniques.
References
Armstrong, L. and VanHeest, J. (2002). The Unknown Mechanism of the Overtraining Syndrome. Sports Medicine, 32(3), pp.185-209.
- Carfagno, D. and Hendrix, J. (2014). Overtraining Syndrome in the Athlete. Current Sports Medicine Reports, 13(1), pp.45-51.
- Fry, R., Morton, A. and Keast, D. (1991). Overtraining in Athletes. Sports Medicine, 12(1), pp.32-65.
Hackney, A. (2006). Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Review of Endocrinology & Metabolism, 1(6), pp.783-792.
- Halson, S. and Jeukendrup, A. (2004). Does Overtraining Exist?. Sports Medicine, 34(14), pp.967-981.
- HOLMÄNG, A. and BJÖRNTORP, P. (1992). The effects of cortisol on insulin sensitivity in muscle. Acta Physiologica Scandinavica, 144(4), pp.425-431.
- Jensen, J., Rustad, P., Kolnes, A. and Lai, Y. (2011). The Role of Skeletal Muscle Glycogen Breakdown for Regulation of Insulin Sensitivity by Exercise. Frontiers in Physiology, 2, p.112.
- Kreher, J. and Schwartz, J. (2012). Overtraining Syndrome. Sports Health: A Multidisciplinary Approach, 4(2), pp.128-138.
- LEPPÄLUOTO, J., HUTTUNEN, P., HIRVONEN, J., VÄÄNÄNEN, A., TUOMINEN, M. and VUORI, J. (1986). Endocrine effects of repeated sauna bathing. Acta Physiologica Scandinavica, 128(3), pp.467-470.
McEwen, B. (1998). Protective and Damaging Effects of Stress Mediators. New England Journal of Medicine, 338(3), pp.171-179.
- Meeusen, R., Duclos, M., Foster, C., Fry, A., Gleeson, M., Nieman, D., Raglin, J., Rietjens, G., Steinacker, J. and Urhausen, A. (2013). Prevention, diagnosis and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science (ECSS) and the American College of Sports Medicine (ACSM). European Journal of Sport Science, 13(1), pp.1-24.
- Olaru, C., Diaconescu, S., Trandafir, L., Gimiga, N., Olaru, R., Stefanescu, G., Ciubotariu, G., Burlea, M. and Iorga, M. (2016). Chronic Functional Constipation and Encopresis in Children in Relationship with the Psychosocial Environment. Gastroenterology Research and Practice, 2016, pp.1-7.
- Ressler, K. (2010). Amygdala Activity, Fear, and Anxiety: Modulation by Stress. Biological Psychiatry, 67(12), pp.1117-1119.