|
Figure 1: Neurokinin 1 Receptor (Gayen et al, 2010) |
Neurokinin Receptor Signaling
Ni Chionnaith, J., Sasitorn, S. and Volkmann, T.
Neurokinin receptors (NKR), also known as substance P receptors (SPR), are an important G protein coupled receptor broadly distributed throughout the central and peripheral nervous systems. Neurokinins are peptides of the tachykinin family that interact with neurokinin receptors. This review discusses neurokinin receptors, their effects on physiological processes and the clinical significance.
Substance P plays a role in the regulation of affective behaviour and emesis in the brain and nociception in the spinal cord. These biological effects are mediated by Substance P binding to the NK receptor. Only a small number of neurons (5-7%) located in certain areas of the central nervous system express the NKR and this SP-NKR system has been the most thoroughly studied of the NK type pathways (Mantyh, 2002). Unstimulated neurons contain the NKR which is distributed in the plasma membrane of the cell bodies and dendrites. When the NKR binds with Substance P it undergoes rapid internalization which is followed by the efficient recycling of the plasma membrane itself. The release of Substance P is as a result of stress stimulus and the quantity released is directly proportional to the intensity and number of stimuli. Stronger intensity and a greater frequency allow for further diffusion of Substance P from the release site resulting in the activation of approximately 3-5 times higher number of NKR-expressing neurons. As an extensively studied receptor there has been recent research into pharmacological applications or the inactivation of the NKR genetically. Due to the vital role that the SP-NKR system plays in the regulation of affective behaviour, this new research suggests that inhibiting the SP-NKR pathway could be a treatment option for depression and associated anxiety (Mantyh, 2002).
Contents
Classification and Types
There are three main types of neurokinin receptors: Neurokinin-1 receptor (NK1R), neurokinin-2 receptor (NK2R) and neurokinin-3 receptor (NK3R). These neurokinin (or tachykinin) receptors are members of the Class I (rhodopsin-like) G-protein coupled receptor (GPCR) family. The tachykinins differ from each other in their affinity to bind with neurokinin receptors. NK1R is mainly involved with substance P (SP). NK2R’s main ligand is substance K (SK) and NKB for NK3R. The neurokinin receptors contain a total of seven transmembrane loops and are classed as members of the transmembrane G-protein coupled receptor (GPCR) superfamily. The neurokinin-1 receptor once in contact with the Gαq-protein and activates phospholipase C which is then followed by the production of inositol triphosphate (IP3) resulting in an increase in the level of intracellular calcium as a second messenger. NK1R coupled with the Gαs-protein also stimulate cyclic AMP (cAMP) (Douglas and Leeman, 2012).
|
Figure 2: Mechanism of Neurokinin Receptor Signaling (Steinhoff et al, 2014) |
Mechanism of Activation
The mechanism and process involved in the SP-NKR system is as follows. Synthesized by neurons and then transported to synaptic vesicles, SP is then released by the depolarization of calcium-dependant mechanisms. Once stimulated these NK1R generate a number of second messengers which in turn trigger various effector mechanisms involved in the function and regulation of cellular excitability. The activation of a first particular secondary messenger system stimulates the production of phosphatidyl inositol via phospholipase C, resulting in the mobilization of calcium from intracellular and extracellular stores. The second stimulates the mobilization of arachidonic acid through the phospholipase A2, and the third results in the accumulation of cAMP through adenylate cyclase stimulation (Douglas and Leeman, 2012). Figure 1 represents a simplified schematic of the mechanism of neurokinin receptor signaling.
Physiological and Clinical Significance:
Nervous System
Substance P is found throughout the CNS, and both neuronal and glial cells have been found to be a source of this neuropeptide (Marriot, 2004). In a study by Nakaya et al, (1994), substance P receptor activity was seen to be mostly localized to neuronal cell bodies and dendrites. CNS glial cells are the most abundant cell type in the CNS. They surround and support neurons, providing protection. They express tachykinin receptors, whose activation has been found to prompt signal transduction pathways and can initiate, or strengthen their inflammatory response (Marriot, 2004).
The tachykinin NK1 receptor is widely distributed in both the central and peripheral nervous systems. In the CNS, their activation regulates respiratory and cardiovascular function, and they play a part in activating the vomit reflex as well as the micturition (urination) reflex. At the spinal cord, the receptors are activated during synaptic transmission (by substance P or tachykinins) in response to harmful stimuli at primary afferent neurons. Through research in neurophysiology as well as behaviour, NK1 receptors have also been found to play a role in pain transmission (Quartara et al, 1998).
In the peripheral nervous system, NK1 receptors are expressed in the gastrointestinal, respiratory and genitourinary tracts. In the respiratory system, they facilitate neurogenic inflammation when the airway is exposed to irritants. In the gastrointestinal system, they regulate water and ion secretion as well as mediating smooth muscle contraction and neuro-neuronal communication. In the cardiovascular system, they mediate endothelium dependent vasodilation and plasma protein extravasation. In the genitourinary tract, they are abundant in the ureter, renal pelvis, urethra and urinary bladder and facilitate inflammation response to harmful stimuli as well as mediating smooth muscle contraction (Quartara et al, 1998).
Immune System
Neurokinin and substance P (SP) are expressed by various immune cells and have inflammatory and immunity function. In mast cells, SP induces the release of TNF (involved in the inflammation response) (Delgado et al, 2003) and vascular endothelial growth factor (Shaik-Dasthagirisaheb et al, 2013), thus contributing to inflammation, immunity and angiogenesis. In monocytes and macrophages, NKR expression is affected by diseases and disorders, becoming upregulated to speed up recovery (Pradhan et al, 2013; Yaraee et al, 2007). SP regulates the activity and function of neutrophils, controlling their production and infiltration into inflamed tissues and promoting bacterial phagocytosis (Augustyniak, et al, 2012; Okaya et al, 2004).
Neurokinin (NK) receptors are also found in eosinophils, and their activation has been found to promote eosinophil recruitment in a model of allergic asthma in mice (Alessandri, et. al., 2003). Dendritic cells coordinate the activation of antigen specific effector and memory lymphocytes, and thus play a major role in acquired immunity. Human and murine dendritic cells have been found to express NK receptors whose activation regulates T-cell function (O’Connell et al, 2005; Kitamura, et al, 2012). Natural killer cells express NK receptors (Feistritzer et al, 2003), and their activation by SP enhances the cytotoxicity of natural killer cells (Lang et al, 2003). NK receptors also have a significant impact on lymphocytes. T lymphocytes produce SP and express NK receptors during infection. SP regulates T-cell proliferation, cytokine and chemokine release, and killer activity (Wang et al, 2006).
Murine B lymphocytes also express neurokinin receptors, and their activation has been found to regulate B-cell maturation and differentiation (Grassin-Delyle et al, 2011; Zhang et al, 2000). Neurokinin has actually been found to have an inhibitory effect on the production of myeloid cells, while SP promotes myelogenesis. This is one of the few instances where the two substances have opposing effects, and this may be an important feedback mechanism to maintain homeostasis (Zhang et al, 2006).
Digestive System
Tachykinins can be found in intestinal immune and enterochromaffin cells although the major sources are enteric neurons, nerve fibres from the dorsal root and ganglia from the vagus group. Enteric neurons, cells of Cahal in the interstitial, epithelial cells and components off the lamina propria (lymphocytes and macrophages) express the NK1 receptor; myocytes, neuronal varicosities and epithelial cells express the NK2 receptor; and NK3 receptor is mostly expressed neuronally (Shimizu et al, 2008). The location of these neurokinin receptors corresponds with the regulation of neuro-neuronal transmissions, secretion, motility, inflammation and pain.
A number of preclinical trials have been carried out showing the possible therapeutic use of antagonists for all three NKRs to treat irritable bowel syndrome (IBS) and related disorders of the gastrointestinal system. One such study carried out on women with diarrhea-predominant IBS who were given a NK1R/NK2R antagonist DNK333 experienced relief from symptoms compared to those on the placebo (Zakko et al, 2011). Similarly, a trial was conducted on female IBS patients to examine the effect of the NK1R antagonist AV608 on visceral pain and anxiety during stimulation using functional magnetic resonance imaging to map the activity of the brain (Tillisch et al, 2012). The use of AV608 resulted in a reduction in both anxiety and pain, suppressing the amygdala, hippocampus and anterior cingulate gyrus regions of the brain throughout visceral distension. While these recent studies have so far only been small-scale, their findings are encouraging and warrant further research into the use of targeted NKR antagonists. Although future clinical applications appear to be promising there are limitations due to the fact that NK1R disruption leads to a delay in the healing of inflamed colons in the treatment of IBD (Steinhoff et al, 2014). Further examination and research is required.
Respiratory System
In the respiratory system, NK1R, located in the endothelial cells of post capillary venules. are activated by SP/NKA which stimulates the extravasation of plasma and the infiltration of granulocytes in the airway (Rodgers, 2011). NKR also affects the airways by stimulating secretion from seromucous glands. After twenty years of intensive research, it was proposed that tachykinins and NK1R play a role in the hyperactive response in the airways of mice that were allergen-stimulated. This suggests that SP and the NK1R play a major role in the hyper-responsiveness of the airway due to allergens (Hens et al, 2011), hence why it has been theorised as a possible treatment for respiratory disorders.
Tachykinins have been shown to contribute to neurogenic inflammation and a hyperactive respiratory system. Due to this influence it has been suggested that NKR antagonists may provide a viable treatment for respiratory disorders such as asthma and bronchitis. When this theory was applied and peptide NK1R antagonist, FK888 was given as a treatment for exercise-induced asthma an improvement was seen. However, the NK1R antagonist CP99,994 gave no bronchial protection to patients with mild asthma. Similarly, while the dual NK1/NK2 receptors antagonist, AVE5883, displayed antagonist behaviour against inhaled NKA, in asthmatics it did not provide any protection against the challenge of allergens (Boot et al, 2007). There is undoubtedly a potential to decrease airway hyper-responsiveness and provide functional improvement to the lung by using these NKR antagonist, although, further research is required to their effects on asthmatic symptoms and inflammation of the airways before conclusions on clinical applications can be made (Ramalho et al, 2011).
Reproduction System
Neurokinin receptor signaling pathways play a significant role in the reproductive system. All 3 types of receptors (NK1R, NK2R, and NK3R) are found in the reproductive organs. Sensory nerves innervation can be found along the renal pelvic wall, ureter, and urinary bladder, which can induce the secretion of substance P (SP). Excess amount of SP secretion in the urinary bladder can cause uncontrolled bladder. Since SP is a ligand for NK1R, NK1R antagonists can be a potential treatment for patients with uncontrolled urination.
According to an article from the American Physiological Society, NK1R also have effects on pro-inflammation including “stimulation of plasma extravasation and leukocyte infiltration, mast cell degranulation, generation of ROS, and expression of proinflammatory cytokines, chemokines, adhesion molecules, and cyclooxygenase-2” (Steinhoff et al, 2014). The pro-inflammatory effects of NK1R can be so severe that it may lead to abortion in pregnancy. NK2R signaling is involved muscle contraction of ureter, where the muscles are substance P containing fibers. The pathway is regulated by ovarian steroids.
In males, NK2R is also suggested to promote sperm motility. Sperm produces an enzyme called “neprilysin”, which degrades substance P. NK2R acts as an inhibitor for the degradation, resulting in activation of sperm motility. On the other hand, NK3R is said to regulate GnRH secretion and thus regulate gonadotropin hormones. However, it is still inconclusive whether the pathway acts as a stimulator or suppressor. Kisspeptin is an activator of puberty. It was observed in experiments, where NKB stimulate the release of kisspeptin, therefore, indirectly stimulate the release of GnRH. However, kisspeptin doesn’t required the stimulation from NKB to be effective, though, NKB cannot stimulate the secretion without kisspeptin. Patients with IHH (low circulating gonadotropin hormones) were administered with GnRH, although, results were inconsistent. In some experiments, the treatment successfully restore the levels of gonadotropin hormone and some suggested the administration of NKB instead (Shao, 2012). The effects of NK3R signaling on GnRH secretion remains inconclusive, however, there are many co-factors that may affect the results, including species, sex, age, gonadal status, and circulating hormones.
|
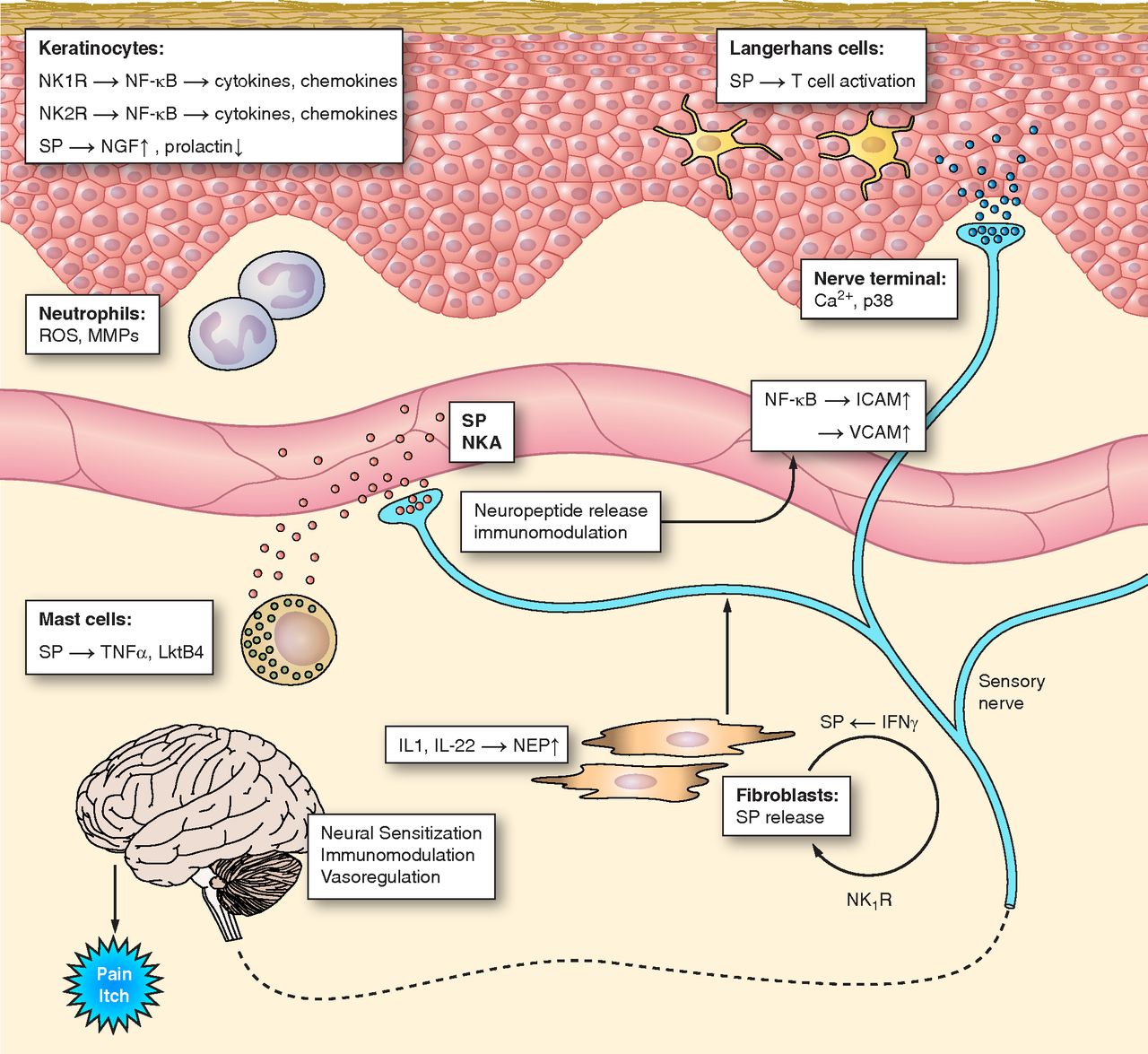
Figure 3: Neurokinin Receptors Signaling on the Skin (Steinhoff et al, 2014) |
Skin
Sensory nerves containing tachykinins innervate dermis (inner layer of skin), epidermis (outer layer of skin), dermal blood vessels, and hair follicles. These tachykinins are mainly Substance P (SP) and NKA or Substance K, which have physiological effects on the skin. There are several factors stimulating their secretion, including physical stimulation, allergens and inflammatory mediators. SP and NKA act as cytokine stimulators, where they regulate the amount of keratinocytes. Keratinocytes are cells in the epidermis, producing pro-inflammatory cytokines. Substance P also up-regulate the expression of nerve growth factor (NGF), which suggests that SP can indirectly promote the regeneration of cutaneous nerves during wound healing. Neurokinin 1 Receptor signaling pathway also promotes plasma extravasation, in which leaks pro-inflammatory mediators.
SP can also activate neutrophils and langerhans cells to amplify the inflammatory response of the skin. With the pro-inflammatory mediators, SP can also causes an itchy sensation, where antagonists of NK1R are used as anti-itchy treatments. Furthermore, SP in sensory nerves innervating hair follicles down regulate the production of prolactin. Apart from stimulating milk production, prolactin also plays an important role in hair growth. This signaling pathway explains hair-loss from stress, where stress stimulate the release of SP.
Conclusion
Neurokinins signaling play essential roles in a number of important physiological processes. Activation of tachykinin receptors in the central and peripheral nervous systems results in signal transduction pathways which initiate or strengthen immune and inflammatory responses, various reflexes, pain transmission, muscle responses, water and ion exchange and neuro-neuronal communication.Various immune cells, which have inflammatory and immunity function, express tachykinin receptors. Substance P regulates the activity and function of neutrophil and eosinophil granulocytes, dendritic cells, B and T lymphocytes, natural killer cells and myeloid cells.
The use of targeted NKR antagonists in the digestive system as a possible treatment for inflammatory bowel syndrome appears quite promising, however there are limitations to this application as it has been shown to delay healing of the inflamed colon by disrupting the NK1R. Similarly, NKR antagonist could also provide an effective treatment for respiratory diseases such as asthma, by reducing the airways hyperactive response. However this application could be limited to certain types of asthma and further development and research is needed.
All 3 neurokinin receptors can be found in the reproductive system. NK1R signaling focuses mostly on the pro-inflammatory effects, where the NK2R signaling is mainly for the muscle contractions of ureter. NK3R plays a more significant role in the gonadotropin hormones secretion. However, the specific effects of NK3R is still inconclusive on whether it acts as a stimulator or inhibitor for the GnRH secretion. Essentially, NK1R antagonists can be used in the treatment of bladder disorders. In conclusion, due to the vital role that NKR play in important physiological processes, the use of NKR antagonists for the treatment of various different disorders could be of clinical significance in the future. However further research is still required before any firm conclusions can be made.
References
Alessandri AL, Pinho V, Souza DG, Castro MS, Klein A, Teixeira MM. (2003). ‘Mechanisms underlying the inhibitory effects of tachykinin receptor antagonists on eosinophil recruitment in an allergic pleurisy model in mice’. Br J Pharmacol 140: 847–854.
Augustyniak D, Jankowski A, Mackiewicz P, Skowyra A, Gutowicz J, Drulis-Kawa Z. (2012). 'Innate immune properties of selected human neuropeptides against Moraxella catarrhalis and nontypeable Haemophilus influenzae'. BMC Immunol 13: 24.
Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, Cohen J, Sterk PJ, Miller B, Paccaly A, Burggraaf J, Cohen AF, Diamant Z. (2007). ‘Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma’. Am J Respir Crit Care Med 175: 450–457.
Chang MM, Leeman SE. (1970). ‘Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P’. Journal of Biological Chemistry. 245:4784–4790.
Delgado AV, McManus AT, Chambers JP. (2003). ‘Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P’. Neuropeptides 37: 355–361.
Douglas SD, Leeman SE. (2011). ‘Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation’. Annals of the New York Academy of Sciences. 2011;1217:83-95. doi:10.1111/j.1749-6632.2010.05826.x.
Ebner, K., N. Singewald. (2006). ‘The Role of Substance P in Stress and Anxiety Responses’. Amino Acids 31. Web.
Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, Kahler CM. (2003). ‘Natural killer cell functions mediated by the neuropeptide substance P'. Regul Pept 116: 119–126.
Gayen A, Goswami SK, Mukhopadhyay C. (2010). 'NMR evidence of GM1-induced conformational change of Substance P using isotropic bicelles'. Biochim.Biophys.Acta.
Grassin-Delyle S, Buenestado A, Vallat L, Naline E, Marx S, Decocq J, Debre P, Bernard OA, Advenier C, Devillier P, Merle-Beral H. (2011). ‘Expression and proliferative effect of hemokinin-1 in human B-cells’. Peptides 32: 1027–1034.
Hens G, Raap U, Vanoirbeek J, Meyts I, Callebaut I, Verbinnen B, Vanaudenaerde BM, Cadot P, Nemery B, Bullens DM, Ceuppens JL, Hellings PW. (2011). ‘Selective nasal allergen provocation induces substance P-mediated bronchial hyperresponsiveness’. Am J Respir Cell Mol Biol 44: 517–523.
Kitamura H, Kobayashi M, Wakita D, Nishimura T. (2012). ‘Neuropeptide signaling activates dendritic cell-mediated type 1 immune responses through neurokinin-2 receptor’. J Immunol 188: 4200–4208.
Kradin R, MacLean J, Duckett S, Schneeberger EE, Waeber C, Pinto C. (1997). ‘Pulmonary response to inhaled antigen: neuroimmune interactions promote the recruitment of dendritic cells to the lung and the cellular immune response to inhaled antigen’. Am J Pathol 150: 1735–1743.
Lang K, Drell TL, Niggemann B, Zanker KS, Entschladen F. (2003). ‘Neurotransmitters regulate the migration and cytotoxicity in natural killer cells’. Immunol Lett 90: 165–172.
Marriott, I. (2004). ‘The role of tachykinins in central nervous system inflammatory responses’. Frontiers in bioscience: a journal and virtual library, 9, 2153-2165.
Mathers, Alicia R., Olga A. Tckacheva, Brian M. Janelsins, William J. Shufesky, Adrian E. Morelli, Adriana T. Larregina. (2007). ‘In Vivo Signaling through the Neurokinin 1 Receptor Favors Transgene Expression by Langerhans Cells and Promotes the Generation of Th1- and Tc1-Biased Immune Responses’. The Journal of Immunology (2007): 7006-017. Web. 05 Apr. 2016.
Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. (2007).’ In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses’. J Immunol 178: 7006–7017.
Mantyh PW. 2002. ‘Neurobiology of substance P and the NK1 receptor’. J Clin Psychiatry. (2002);63 Suppl 11:6-10.
Nakaya, Y., Kaneko, T., Shigemoto, R., Nakanishi, S., & Mizuno, N. (1994). ‘Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat’. Journal of Comparative Neurology, 347(2), 249-274.
O’Connell PJ, Pingle SC, Ahern GP. (2005). ‘Dendritic cells do not transduce inflammatory stimuli via the capsaicin receptor TRPV1’. FEBS Lett 579: 5135–5139.
Okaya T, Holthaus R, Kato A, Lentsch AB. (2004). ’Involvement of the neuropeptide substance P in lung inflammation induced by hepatic ischemia/reperfusion’. Inflamm Res 53: 257–261.
Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, Chun M, Auster ME, Nabzdyk C, Deso S, Andersen N, Gnardellis C, Logerfo FW, Veves A. (2013). ‘Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing’. J Vasc Surg 58: 766 –775.
Ramalho R, Soares R, Couto N, and Moreira A. (2011). ‘Tachykinin receptors antagonism for asthma: a systematic review’. BMC Pulmonary Med 11: 41.
Rogers DF. (2011). ‘Motor control of airway goblet cells and glands’. Respir Physiol 125: 129– 144.
Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Potalivo G, Caraffa A, Antinolfi P, Tete S, Tripodi D, Conti F, Cianchetti E, Toniato E, Rosati M, Conti P, Speranza L, Pantalone A, Saggini R, Theoharides TC, Pandolfi F. (2013). ‘Vascular endothelial growth factor (VEGF), mast cells and inflammation’. Int J Immunopath Pharmacol 26: 327–335.
Shao, Paul Peng Lin. (2012). 'The Functional Significance of Neurokinin 3 Receptor Signaling in Gonadotropin Releasing Hormone Neurons'. Electronic Theses and Dissertations UC San Diego: 1-86. Web. 06 Apr 2016.
Shimizu Y, Matsuyama H, Shiina T, Takewaki T, and Furness JB. (2008). ‘Tachykinins and their functions in the gastrointestinal tract’. Cell Mol Life Sci 65: 295–311.
Smyth CM, Akasheh N, Woods S, Kay E, Morgan RK, Thornton MA, O’Grady A, Cummins R, Sheils O, Smyth P, Gleich GJ, Murray FM, Costello RW. (2013). ‘Activated eosinophils in association with enteric nerves in inflammatory bowel disease’. PLoS One 8: e64216.
Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. (2014). ‘Tachnykinins and their receptors: contributions to physiological control and mechanisms of disease’. Physiol Rev 94: 265–301.
Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, Siffert J, McKelvy J, Naliboff B, Mayer E. (2012). ‘Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study’. Aliment Pharmacol Therapeut 35: 360 –367.
Quartara, L., & Maggi, C. A. (1998). ‘The tachykinin NK 1 receptor. Part II: Distribution and pathophysiological roles’. Neuropeptides, 32(1), 1-49.
von Euler US and Gaddum JH. (1932). ‘An unidentified depressor substance in certain tissue extracts’. J Physiol (Lond) 1932;72:577–583.
Wang X, Douglas SD, Peng JS, Zhou DJ, Wan Q, Ho WZ. (2006). ‘An in vitro model of morphine withdrawal manifests the enhancing effect on human immunodeficiency virus infection of human T lymphocytes through the induction of substance P’. Am J Pathol 169: 1663–1670.
Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F, Ghazanfari T. (2007). ‘The effect of substance P on nitric oxide production by HSV-1 infected macrophages’. Int Immunopharmacol 7:135–139.
Zakko S, Barton G, Weber E, Dunger-Baldauf C, and Ruhl A. (2011). ‘Randomised clinical trial: the clinical effects of a novel neurokinin receptor antagonist, DNK333, in women with diarrhoea-predominant irritable bowel syndrome’. Aliment Pharmacol Ther 33: 1311– 1321.
Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. (2002). ‘Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis’. Nat Immunol 1: 392–397.
Zhang, Y., Berger, A., Milne, C. D., & Paige, C. J. (2006). ‘Tachykinins in the immune system’. Current drug targets, 7(8), 1011-1020.