Oncolytic Virus Therapy
Rhiannon Rodgers, Anushya Chandran, Victoria van Eijk
Contents
Introduction
Basics of Oncolytic Virotherapy
At the very core of Oncolytic Virotherapy lies the natural affinity for viruses to infect and replicate within cancerous cells. Malignant cells undergo changes during oncogenesis that enable it to resist programmed apoptosis, growth suppression, and type 1 interferon (IFN) signaling. These characteristics are highly sought out by most viruses, making the two a pair with incredible potential for oncologists. While a great number of viruses have a natural propensity to infect tumor cells, others can be genetically modified to select for cancerous cells, given a large enough genome to effectively insert desirable genes (Filley and Dey, 2017).
History
Early cases in the 19th century observed tumor regression through some naturally acquired viral infections. It was noted that cancer patients would occasionally go into remission after acquiring a viral infection, especially in the case of influenza infecting patients with leukemia. Although by no means providing a complete cure, this discovery kickstarted interest in the usage of viruses as a cytotoxic cancer treatment. Unfortunately, attempts to deliberately infect tumor patients often resulted in antiviral immunity, due to the host immune system clearing the infection before any beneficial change could take place; unless in the case of immunosuppressed patients. However, the high fatality rate of this method rendered it crude and unsuitable for use (Kratzky and Patel, 2013).
Forms of Cancer Treatment
Surgery, radiotherapy and chemotherapy have otherwise been the primary therapies used to treat cancers in the past century. Roentgen’s discovery of x-rays and Curie’s discovery of radiation drastically changed the oncologic field and catapulted these new findings into the spotlight. Interest in virotherapy receded into the background, especially due to its notable shortcomings at the time. However, while radio- and chemotherapies each had significant findings, the inefficacy of both methods when faced with unique cancers, as well as individual flaws, eventually turned some attention back to oncolytic virus (OV) research. As standard virus research progressed, knowledge of their usage in therapy unfurled simultaneously. Soon, viruses became the most widely and wholly understood organism in nature. This knowledge, however, served to highlight the difficulties with OV therapy with even greater intensity. A few constants were understood with each subsequent trial; firstly, that under the proper circumstances, viruses were indeed shown to be cytotoxic to malignant cells while excluding benign host cells. Secondly, regression was most often and most drastically seen in young patients with suppressed immune systems, e.g. patients with leukemia. However, while they were most responsive to these treatments, they were also more likely to cause hemorrhaging and fatal neurotoxicity. Thirdly, it was often found that remissions caused by virotherapy were often incomplete and short-lived (Kelly and Russel, 2007).
Discernible Issues
These key observations raised many essential questions to fuel future OV research. Many of these questions are still under consideration in the modern day. Which viruses are best for treating tumors? Can viruses that exclusively infect other species be used in foreign hosts to avoid fatal side effects? In what other ways can these side effects be avoided? Is it possible to avoid antiviral immune responses so that the virus may shed cancerous cells without being eliminated? How can a particular virus be chosen for a unique cancer? By what mechanisms do the viruses work and how can specific pathways be manipulated for each case? Through the years, these uncertainties about virotherapy have prevented it from becoming a primary option for cancer treatment. While many of these questions have been at least partially answered, much remains a mystery. The currently known nuances of this unique form of therapy will be explored in depth, to shed light on how past successes have shaped the field today, how exactly it functions, and what difficulties have presented themselves.
Notable Experiments
In order to highlight the shortcomings as well as developments of OV treatment, attention must be drawn to experiments done in many years past. Four in particular stand out as significant cornerstones of research. The first, conducted in 1949, used an unpurified wild-type strain of Hepatitis B virus as the sole treatment for 22 patients with Hodgkin’s disease- a cancer that affects the lymphatic system. As it marked one of the earlier trials of virotherapy, many of the patients unfortunately developed hepatitis as an unintended consequence, while only 4 of the 22 ended up with a reduction in the size of the lymphoma. The subsequent trials found a greater margin of success, albeit somewhat slowly- improving at the same rate as standard virus research unfolded.
Three years later, the second notable experiment used an isolate of West Nile Virus named Egypt 101. Such flaviviruses were oft used in trials in early OV research. This trial in particular was conducted with 34 patients with advanced neoplastic diseases. Only 4 of the 30 experienced any tumor regression, and while many of the patients were infected, only two cases of encephalitis were reported.
The third experiment had, however, found far greater success with an adenovirus. Specifically, adenoidal-pharyngeal-conjunctival virus (APC). The 1956 study found that 26 out of 40 patients with a cervical carcinoma showed localized tissue necrosis, and very few developed side effects.
The final key cornerstone experiment, done in 1979, found similar improved success rates to the previous one, with only 11 of 90 patients with terminal cancers showing unresponsive results to inoculation with a wild-type mumps virus. 37 of the 79 patients that exhibited some sort of response had shown complete tumor regression.
These experiments shed essential insight onto equally essential questions that the field had struggled with at the time. Primarily that adenoviruses, herpesviruses, paramyxoviruses, picornaviruses, and poxviruses are the preferred viruses to use and modify for therapies due to milder side effects, especially compared to other viruses, as well as many having a large enough genome to be edited with relative ease (Kelly and Russel, 2007).
Regarding these very side effects, infections can still often occur in virotherapy, but the host immune system is often effective enough to eliminate larger threats, especially when fatal consequences are largely removed from the equation. In the case of immunosuppressed patients, however, this matter is a more delicate one.
Finding a Suitable Virus
Viruses of Foreign Host Origin
To first potentially answer the growing issue that had become the method with which to treat these immunosuppressed patients, the task of finding a suitable virus had been outsourced to viruses of non-human origin. In theory, this would eliminate the concern of severe or fatal secondary effects, as the virus would be unable to replicate uncontrollably in a foreign host. However, this theory does not eliminate the possibility of a mutation; a mutation that could potentially become not only harmful to the patient, but highly infectious as well. Introducing a newly formed virus into a population that had not developed any form of resistance to it would be far too risky, and scientists concluded that the risk would outweigh any potential benefits. Feline panleukopenia virus is one of the primary examples of this phenomenon; it had been previously used in virotherapy before evolving into a new, wild-type form— suddenly transmissible to dogs. This led to the infamous canine parvovirus pandemic of the late 1970s. (Kelly and Russel, 2007)
Newcastle Disease Virus and Vesicular Stomatitis Virus
Not all hope is lost for viruses of foreign origin, however. Viruses such as Newcastle Disease Virus (NDV) and Vesicular Stomatitis Virus (VSV), among others, are strikingly excellent at oncolysis as well as remaining relatively safe to use. Both viruses have a common symptom: conjunctivitis. Inflammation of the eye’s conjunctiva, while unpleasant, is noteworthy due to the incredibly low likelihood of any mutations occurring that would allow the virus to become lethal. NDV is able to cause remarkably efficient decreases in tumor size as well as remissions often lasting past 10 years, with very few detrimental symptoms (Zamarin and Palese, 2012).
VSV is found to be similarly impotent, with a mechanism that expressly targets interferon production— decreasing encephalitis risk tenfold (Kelly and Russel, 2007).
Both viruses have also proven themselves useful as vaccine transmission vectors as well as oncolytic agents (Parks, 2017). While rare, viruses traditionally found in nonhuman hosts can have incredible curative effects.
Mechanism of Action
Wild and Modified Viruses
To further examine just how much this particular treatment option differs from current cancer therapies, an analysis of the functioning pathway must be done. Oncolytic viruses have two primary roles: To target and eradicate cancerous cells, and to induce specific antitumor immunity. The former is naturally the most widely sought-after effect of the viruses.
Traditionally, prior to modern genetic engineering and biotechnology, viruses used in cancer treatment were of a wild-type. While these often had stronger tumor eliminating effects and lower viral toxicity, the frequency and severity of secondary effects make the clinical application too unstable. Genetically modifying wild-type viruses opens the possibilities to avoid a systemic immune response, as engineering allows the virus to more accurately remain within the tumor—where antiviral immune cells are somewhat inhibited from entering.
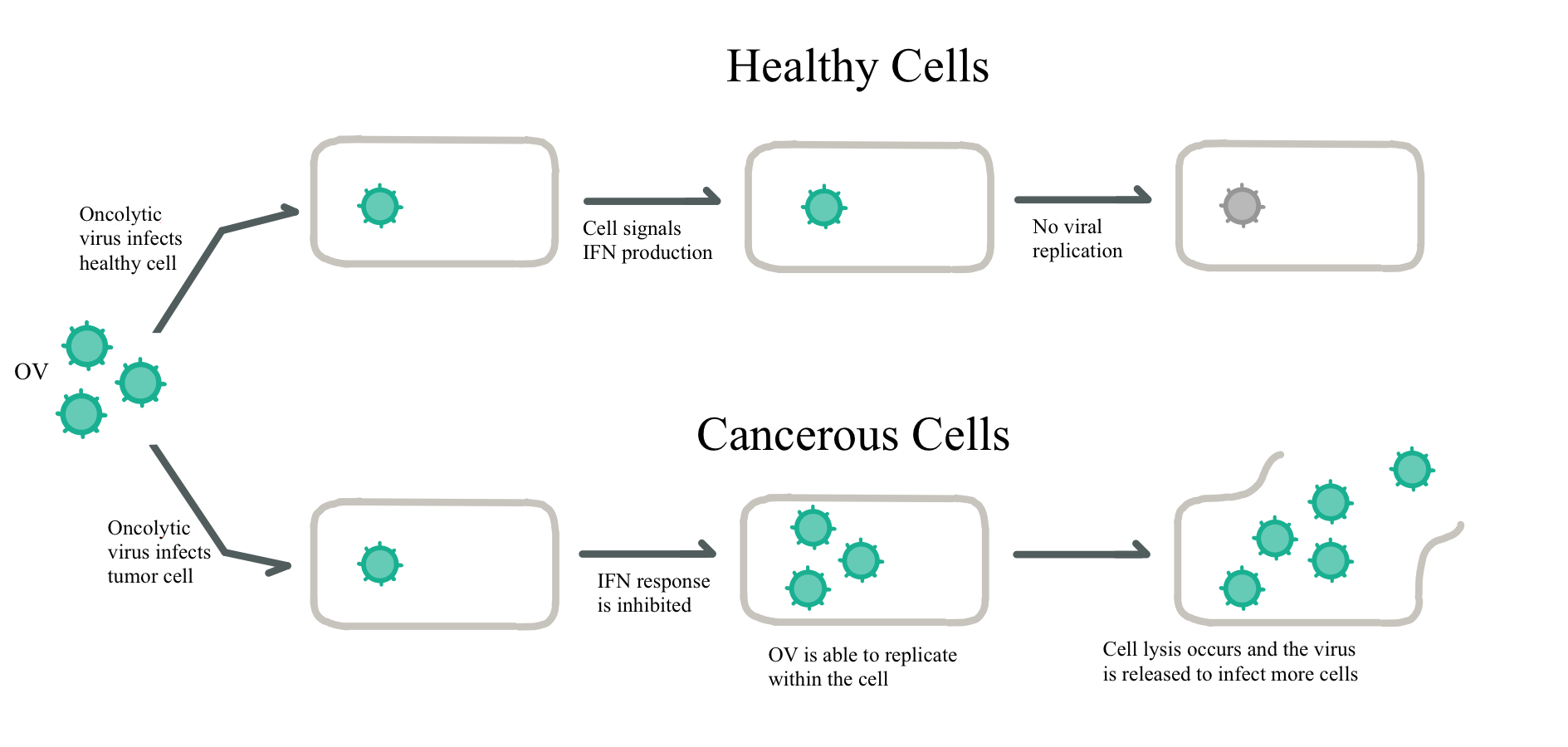
OVs, both wild and modified, typically operate under one or two of three possible mechanisms. The first is the direct lysis of unhealthy cells. Typically, this involves a mixture of apoptosis, necrosis, pyroptosis and autophagic cell death, often with one form being dominant in a particular OV (Bartlett et al, 2013). Furthermore, the direct method utilizes the inactivation of antiviral responses, as seen in Figure 1.
The second method involves the indirect killing of uninfected tumor cells through— in the case of VSV and vaccinia virus— compromising blood flow through cancerous tissue, causing widespread necrosis within the unhealthy, yet uninfected tissue. (Breitbach et al, 2007).
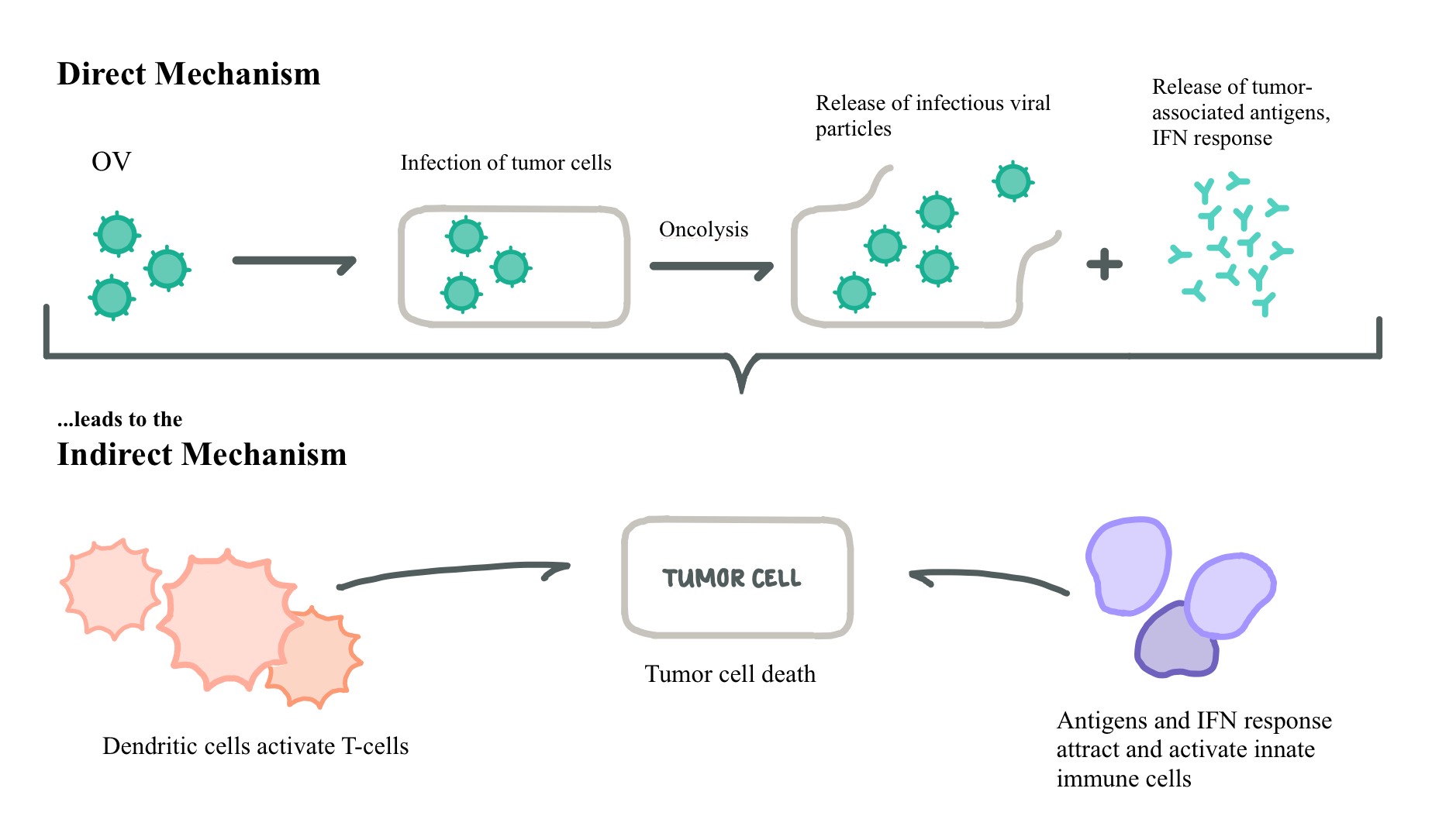
The third mechanism is an indirect method in which cytotoxicity to tumor cells is caused through activation of tumor-specific immune cells, and not from the virus itself. This is shown alongside an abridged view of the direct mechanism in Figure 2 below (Bartlett et al, 2013).
|
Figure 1. Responses to Viral Infection in Normal and Cancerous Cells:Cancer cells disable natural interferon (IFN) production, thus allowing oncolytic viruses to replicate uninhibited, while normal cells abrogate further replication. Adapted from Bell and McFadden, (2014).
-
|
Figure 2. Direct and Indirect Mechanisms of Oncolysis: After a direct mechanism is performed, an indirect response is often seen as a response to the released viral antigens. This often leads to a strong interferon (IFN) response, especially in the cases of double-stranded RNA viruses. Adapted from Alemany and Cascallo, (2009).
Newcastle Disease Virus; Mechanism of Action
NDV in particular has a mechanism of action that is especially effective in cancer cells. As it is a type of paramyxovirus, the genome consists of single-stranded RNA. When NDV infects a healthy somatic cell, the single-stranded RNA replicates into a double strand of RNA. As double stranded RNA does not occur naturally anywhere in the host body, a strong interferon response is typical. However, in a tumor cell, the defection of the protein kinase PKR pathways— that activate the interferon signaling pathway— results in no antiviral response, thus increasing the efficacy of the virus (Bai et al, 2019).
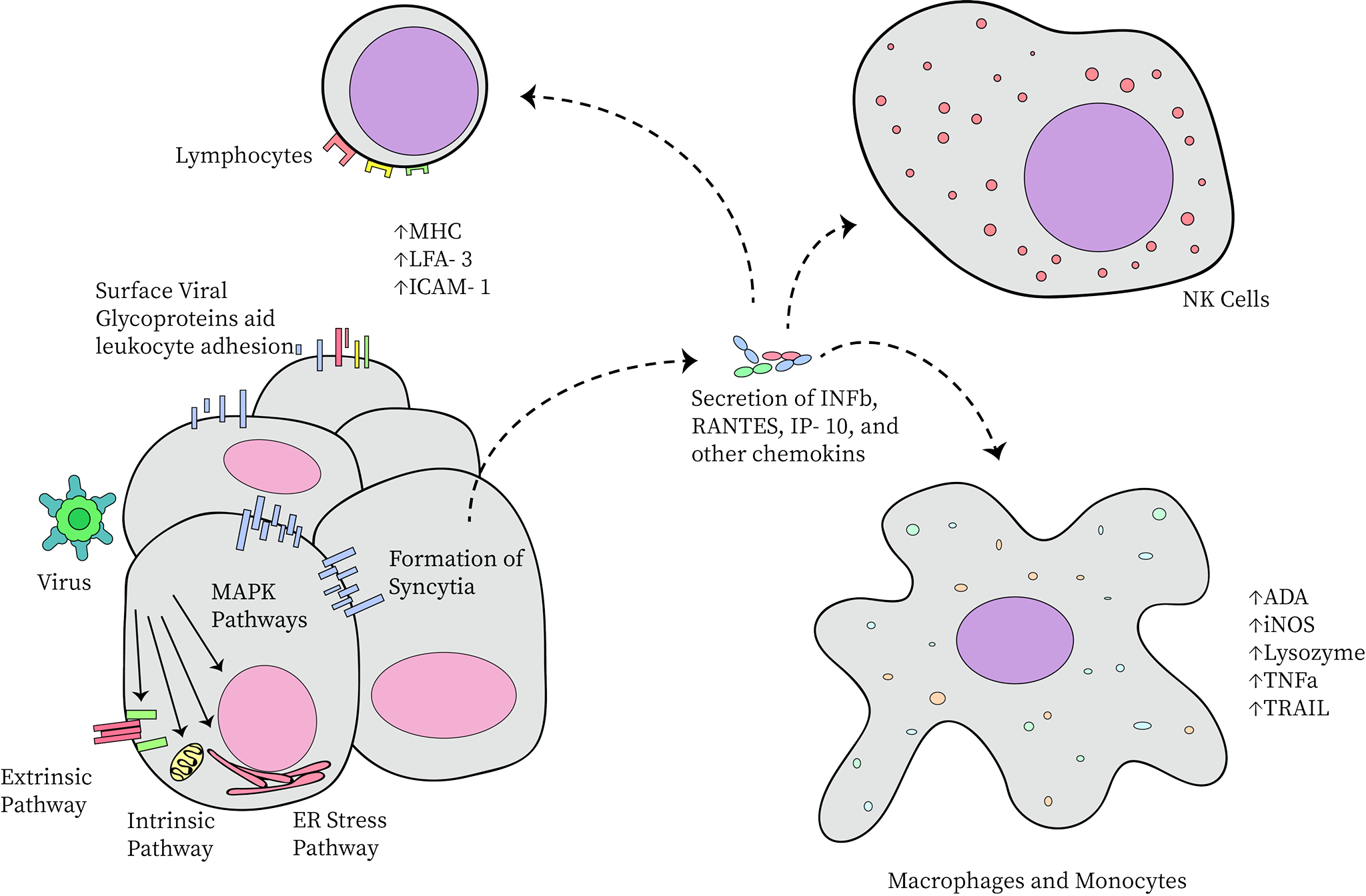
Directly, NDV forms multinucleated syncytia, allowing the virus to spread further undetected by the immune system. It also activates the extrinsic and intrinsic apoptotic pathways, the ER stress pathway, and the MAPK pathways. Each of these involves a protein kinase cascade in which the end result is some form of cytolysis or viral propagation—typically both. Indirectly, NDV induces the secretion of inflammatory cytokines. This includes the activation of both innate and adaptive immune responses (including, but not limited to lymphocytes, macrophages, and standard antibody-producing cells). The indirect antitumor response is further intensified by increased leukocyte adhesion to viral glycoproteins present in the membrane of unhealthy cells. This immune cell binding is stimulated through the release of specialized adhesion-promoting hormones. These hormones also induce the production of tumor-specific lymphocytes and macrophages (Zamarin and Palese, 2012). Visually, this is demonstrated in Figure 3 below.
|
Figure 3. Specific Forms of Cytotoxicity Utilized by Newcastle Disease Virus: MHC: major histocompatibility complex, LFA-3: lymphocyte function-associated antigen-3, ICAM-1: intercellular adhesion molecule-1, IFNβ: interferon β, RANTES: regulated upon activation, normal T cell expressed and secreted, IP-10: interferon gamma-induced protein-10, ADA: adenosine deaminase, iNOS: inducible nitric oxide synthase, TNFα: tumor necrosis factor α, TRAIL: TNF-related apoptosis-inducing ligand (Zamarin and Palese, 2012).
Reovirus; Mechanism of Action
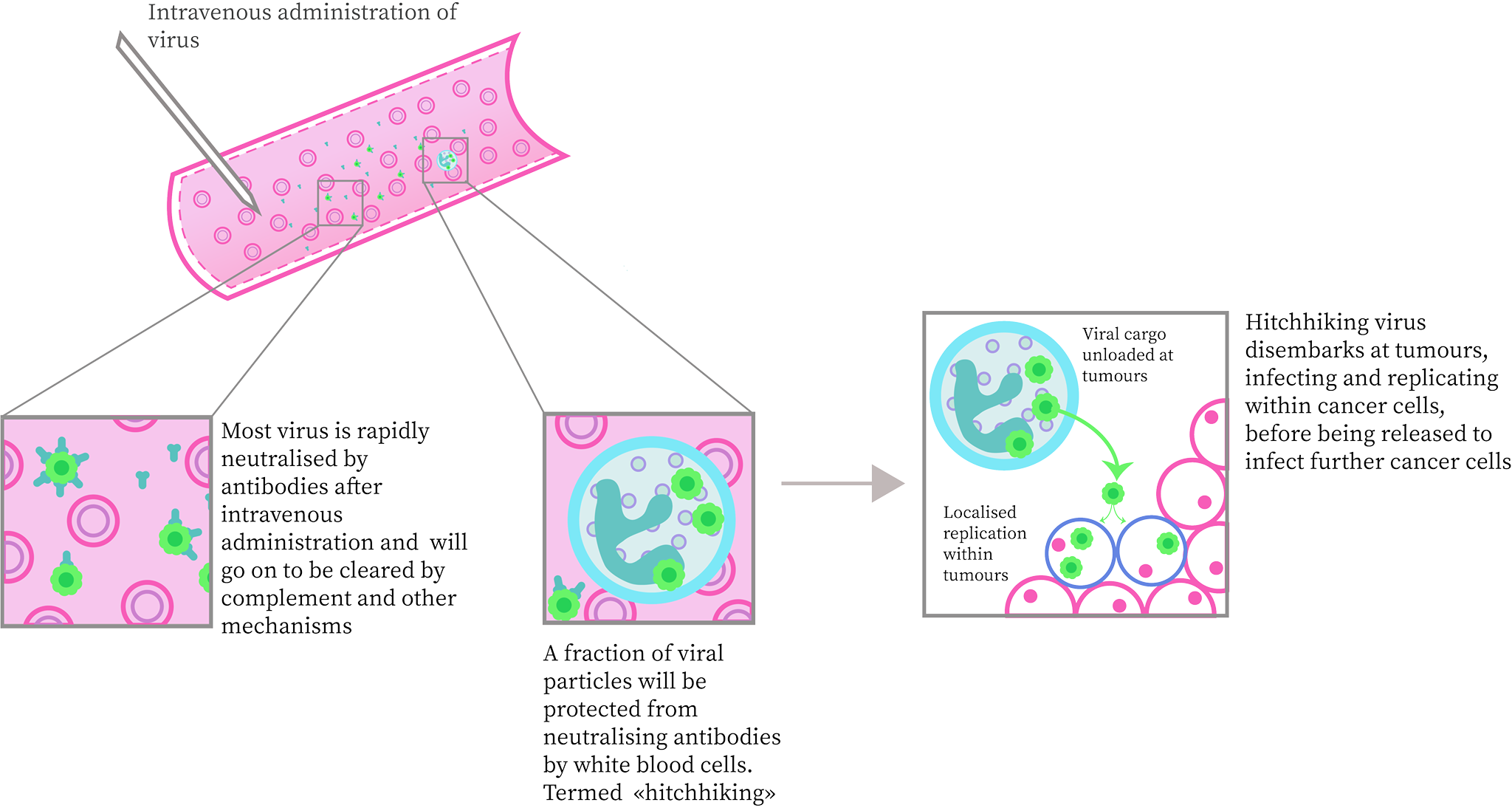
Reoviruses, another oft used OV, avoids detection in the body by latching onto white blood cells. This prevents a direct antiviral response, as seen in Figure 4. The virus is then deposited to the cancerous cells where it proceeds to replicate within cells with Ras-activated signaling pathways. This Ras-pathway produces a phospholipase that promotes replication in certain RNA viruses (such as reovirus). Due to this specificity constraint, an oncolytic virus such as certain reoviruses should only be selected for use in tumors that have a high occurrence of Ras signaling pathways (Bai et al, 2019).
|
Figure 4. Mechanism by which Reovirus avoids Immune Detection: Reovirus adheres to white blood cells, avoiding complete detection within the bloodstream until being deposited to the site of the tumor. From there it carries out standard direct and indirect oncolysis methods. Adapted from Bell and McFadden, (2014).
The Immune System in Relation to Virotherapy
Immunosuppression
Within the core nature of a tumor lies its ability to restrict the activity of antiviral immune cells, both specific and innate. This, along with the inhibition of forced apoptosis, and the endless production loop of cell growth promoters, give cancer its uniquely characteristic unlimited expansion capabilities. This is, in part, due to the reduced function of immune cells— namely the T-cells— to enter and lyse the tumor, as they might with traditionally malignant cells. This is primarily why, in a tumor, viruses are needed to replace the role that the immune system traditionally has for unhealthy, non-cancerous cells (Cervera-Carrascon et al, 2020).
The dependence on the host immune system stands antinomically to the idea that the same system exists to eradicate the very viruses that attempt to treat it. What methods can be employed to reduce the efficacy of the host immune system in order to allow the oncolytic agent to perform its intended task, without exposing the patient to undue harm? A potential candidate to clarify this issue is the compound cyclophosphamide (CPA). A related study concluded that exposure to CPA, even briefly, suppressed antiviral responses within the host. Longer exposure to CPA impeded neutralizing antibody activity. Furthermore, the study confirmed that CPA treatment of rats with significant intracerebral tumors considerably increased the propagation and longevity of the administered treatment virus, eventually leading to neoplastic regression (Ikeda et al, 1999).
Immunocentrism and Virocentrism
The notion of the immune system being both antagonistic and complementary to virotherapy has divided the field into opposing tracts; an immunocentric, and a virocentric view. The immunocentrists argue that a symbiosis between the body and the virus is the optimal strategy to adopt when considering treatment options, as well as the future of virotherapy. This perspective suggests the utilization of induced immune effector cells as a way of lysing disseminated tumor cells around the affected organ, where it is out of reach of the OV. One drawback to this viewpoint is the existence of tumor-generated localized immunosuppression, and thus each region of the tumor must then be accessible to the oncolytic virus (Alemany and Cascallo, 2009).
Conversely, virocentricity understands the immune system as hindering the effects of an OV, and seen as an obstacle to be overcome. The immunogenic quality of a virus is so strong that it dominates all elicited immunity, impeding a response towards tumor antigens. Immunosuppression is thus seen as exclusively advantageous, allowing an unobstructed location for viral replication. The downfall here is the virus itself; the specificity must be so great and so unquestionable in order for it to be considered for medicinal use. For this reason, immunocentrics are favored, yet results prove that regression is most often documented in immunocompromised patients (Alemany and Cascallo, 2009).
Current Research; Genetic Engineering
Talimogene Laherparepvec [T-VEC]
It wasn’t until the progression in reverse genetic science that oncolytic virotherapy improved drastically. This breakthrough allowed new, more highly specific tumor-targeting OVs to enter the field. Perhaps one of the most notable viruses in recent years is the talimogene laherparepvec virus, or T-VEC. T-VEC is a strain of herpes-simplex 1, and has found great triumph in treating melanomas. With an incredibly high success rate and very few side effects, the virus became the first OV approved for clinical use by the FDA in the United States, after completing phase III trials. The virus codes for granulocyte/macrophage colony stimulating factor (GM-CSF) which is especially powerful in priming and amplifying anticancer immunity.
Indirect methods such as these prove to be especially reliable in reducing the risk of cytotoxicity in healthy cells, and improving the patient’s own immune response to further potential relapses—effectively increasing chances of complete remission. Due to this excellent synergy with immunotherapy, T-VEC is an exemplary pioneer for further strains of oncolytic viruses that can be used alongside other therapies (Russell et al, 2014).
Simultaneous use in immunotherapy
Having proven its synergistic capabilities, OVs are currently under testing as tools for simultaneous use alongside certain T-cell therapies. As a standalone treatment, T-cell therapy is ineffective with regards to eradication of malignant cells, due to the inherent nature of tumors. T-cells cannot enter unhealthy cells, nor can they shut down their replicative ability. However, when used alongside a virus engineered to target that particular area, many of the obstacles barring T-cell therapy from effective use are removed. A recent study concludes that the tumor-infiltrating lymphocytes (TIL) had a 62% efficacy when paired with an oncolytic adenovirus, as opposed to a 17.5% response rate when simply paired with a physiological saline solution. A clinical trial of this method is underway as of April of 2020, the results of which, if positive, could shape OV treatment for the foreseeable future (Cervera-Carrascon et al, 2020).
While the field of oncology has long been established, this particular subsect finds itself in a state of constant improvement, of constant directional tide of development. Each subsequent clinical and experimental trial in the past has made the potential of practically applying these methods increasingly clear. However, more extensive research and several more clinical trials are required before we can realistically implement them. Furthermore, the issue of adequate funding and ethical trial procedures still seem to pose an unyielding barrier before proper utilization.
Many of the questions posed at the beginning of the article have been answered, at least in part, but clarifications and consistency in answers are needed before oncolytic virotherapy can realize its true potential alongside other formidable cancer treatments known today. Optimal solutions to simplification of OV selection for treatment without having to sequence an entire genome, or resolving the double-edged relationship between immune cells and OVs are just a few examples of the uncertainties that must be rectified before OV therapy can truly make a breakthrough in treatment of the constantly mystifying disease that is cancer.
References
Alemany, R.; Cascallo, M. (2009). Oncolytic viruses from the perspective of the immune system. Future Microbiology 4(5): 527-36. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19492964
Bai, Y., Hui, P., Du, X., & Su, X. (2019). Updates to the antitumor mechanism of oncolytic virus. Thoracic cancer, 10(5), 1031–1035. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6501037/
Bartlett, D. L.; Liu, Z.; Sathaiah, M.; Ravindranathan, R.; Guo, Z.; He, Y.; Sheng Guo, Z. (2013): Oncolytic viruses as therapeutic cancer vaccines. Molecular cancer 12: 103. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3847443/
Bell, J.; McFadden, G. (2014): Viruses for tumor therapy. Cell host and microbe 15 (3): 260-265. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3963258/
Breitbach, C. J.; Paterson, J. M.; Lemay, C. G., Falls, T. J.; McGuire, A; Parato, K. A; Stojd, D. F.; Daneshmand, M.; Speth, K.; Kirn, D.; McCart, J. A.; Atkins, H.; Bell, J. C. (2009). Targeted Inflammation During Oncolytic Virus Therapy Severely Compromises Tumor Blood Flow. Molecular Therapy, Volume 15, Issue 9, Pages 1686-1693. Retrieved from https://www.sciencedirect.com/science/article/pii/S1525001616320342
Cervera-Carrascon, V.; Quixabeira, D. C.A.; Havunen, R.; Santos, J. M.; Kutvonen, E.; Clubb, J. H.A.; Siurala, M.; Heiniö, C.; Zafar, S.; Koivula, T.; Lumen, D.; Vaha, M.; Garcia-Horsman, A.; Airaksinen, A. J.; Sorsa, S.; Anttila, M.; Hukkanen, V.; Kanerva, A.; Hemminki, A. (2020). Comparison of Clinically Relevant Oncolytic Virus Platforms for Enhancing T Cell Therapy of Solid Tumors. Molecular Therapy Oncolytics, Volume 17, 26 June 2020, Pages 47-60. Retrieved from https://www.sciencedirect.com/science/article/pii/S2372770520300243
Donelly, O.; Harrington, K.; Melcher, A.; Pandha, H. (2013): Live viruses to treat cancer. Journal of the royal society of medicine 106 (8): 310-314. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3725859/
Filley, A. C.; Dey, M. (2017). Immune System, Friend or Foe of Oncolytic Virotherapy?. Frontiers in oncology, 7, 106. Retrieved from https://doi.org/10.3389/fonc.2017.00106
Ikeda, K.; Ichikawa, T.; Wakimoto, H. et al. (1999). Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med 5, 881–887. Retrieved from https://www.nature.com/articles/nm0899_881#Abs1
Kelly, E.; Russell, S. J. (2007): History of oncolytic viruses: Genesis to genetic engineering. Molecular therapy 15 (4): 651-659. Retrieved from https://www.sciencedirect.com/science/article/pii/S1525001616313314l?via%3Dihub
Kratsze, R. A.; Patel, M. R. (2013). Oncolytic virus therapy for cancer: the first wave of translational clinical trials. Gene Therapy for Human Disease: Clinical Advances and Challenges, Translational Research, Volume 161, Issue 4, Pages 355-364. Retrieved from https://www.sciencedirect.com/science/article/pii/S193152441200446X?via%3Dihub
Parks, C. L. (2017) Chapter 2 - Replication-Competent Viral Vectors for Vaccine Delivery. Emerging Technologies in Design and Development, Pages 25-63. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780128023020000017
Raja, J.; Ludwig, J. M.; Gettinger, S. N.; Schalper, K. A.; Kim, H. S. (2018): Oncolytic Virus immunotherapy: Future Prospects for Oncology. Journal for Immunotherapy of cancer 6. Retrieved from https://jitc.biomedcentral.com/articles/10.1186/s40425-018-0458-z
Russell, S. J.; Peng, K.; Bell, J. C. (2014): Oncolytic virotherapy. Nature biotechnology 30 (7): 658-670. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3888062/
Russell, L.; Peng, K. (2018): The emerging role of oncolytic virus therapy against cancer. Chinese clinical oncology 7 (2): 16 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6557159/
Wollmann, G., Ozduman, K., & van den Pol, A. N. (2012). Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer journal (Sudbury, Mass.), 18(1), 69–81. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3632333/
Zamarin, D., & Palese, P. (2012). Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiology, 7(3), 347–367. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4241685/
Figures
Figure 1: Responses to Viral Infection in Normal and Cancerous Cells; drawn by Rhiannon Rodgers, adapted from Bell and McFadden, (2014).
Figure 2: Direct and Indirect Mechanisms of Oncolysis; drawn by Rhiannon Rodgers, adapted from Alemany and Cascallo, (2009)
Figure 3: Specific Forms of Cytotoxicity Utilized by Newcastle Disease Virus; drawn by Anushya Chandran, adapted from Zamarin and Palese, (2012)
Figure 4: Mechanism by which Reovirus avoids Immune Detection; drawn by Anushya Chandran, adapted from Bell and McFadden, (2014)