P53: The Key to Sunbathing (The Regulation of MSH)
An Introduction to P53
This essay discusses the effects of P53, its relation to sunbathing and the tanning response. Additionally, we will be discussing the P53’s relation to cancer and its prognostic factor and the role of MSH.
Fig 1.
The Structure of P53 (Splettstoesser)
Contents
P53 (TP 53) is a tumor suppressor gene that is considered one of the most frequent targets for gene alterations relating to cancer. (Moshe Oren et al, 2007) It codes for a protein that regulates the cell cycle, functioning as a tumor suppressor. In 1993 P53 was voted molecule of the year by science magazine, and has been described as “the guardian of the genome”, relating to ability to prevent genome mutation. In over half of human tumors we can see the inactivation of P53. Due to many experiments on the biochemistry an physiology of P53 relating to its cancer role, it is now appreciated that its main role is to prevent the emergence of cells with permanently defective genomes, which are very likely to lead to cancer. In normal cells, P53 protein is low, but after DNA damage or other stress such as UV radiation, a trigger response may occur, increasing the number of P53 proteins in the cell. These proteins have three major functions: apoptosis (cell death), DNA repair and growth arrest – stopping the cell cycle and therefore preventing replication of damaged DNA. Apoptosis would be a final result to prevent replication of damaged DNA and avoiding tumor growth (Fig.2). Although high levels of P53 can be considered a good thing when referred to cancer suppression, too much can result in accelerated ageing which will be discussed further as an effect of excessive UV radiation.
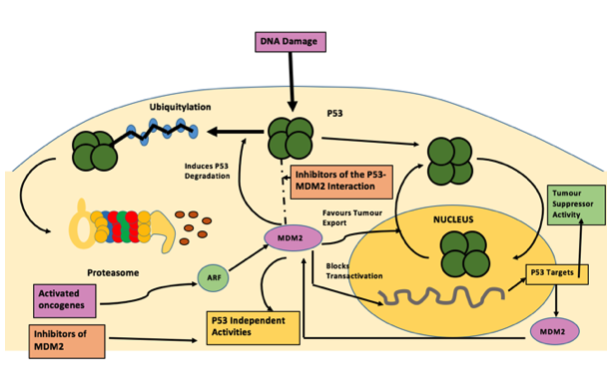
Fig 2.
Figure 2 : Inhibiting the p53–MDM2 interaction: an important target for cancer therapy : Nature Reviews Cancer (Chéne)
Role of P-53 and relation to MSH
P53 is a tumour suppressor gene and is located on the seventeenth (17p13) chromosome. It is one of the most frequent targets for genetic alteration in cancer. A lot of human tumours are able to mutationally inactivate p53, removing its role as a tumour suppressor and preventing the emergence of cells with permanently defective genomes, which can often cause cancer. P53’s ability is largely due to it being able to act as a sequence special c transcriptional regulator. (Moshe Oren et al, 2007) It is also a key regulator of cellular responses to geno-toxic stress, and it is stabilised and activated after DNA damage. The rapid activation of p53 by ionising radiation mostly dependents on the ATM kinase. P53 is phosphorylated by ATM shortly after DNA damage, resulting in enhanced stability and activity of p53. The Mdm2 oncoprotein is a major negative regulator of P53, triggering its degradation by ubiquitin system (Fig. 3). In response to ionising radiation and radio-mimetic drugs, Mdm2 undergoes rapid ATM-dependent phosphorylation before to p53 accumulation. This decreases its reactivity with the 2A10 monoclonal antibody. Phage display analysis is a laboratory technique, for the study of proteins and protein-DNA that use bacteriophages to connect proteins with the genetic information. From this analysis a consensus 2A10 recognition sequence was identified, possessing the core design DYS. Unexpectedly, this design appears twice within the human Mdm2 molecule, at positions corresponding to residues 258–260 and 393–395. Serine 395, residing within the carboxy-terminal 2A10 epitope, is the major target on Mdm2 for phosphorylation by ATM in vitro. (Ruth Maya et al, 2001) Alpha-Msh is a a-melanocyte-stimulating hormone secreted by keratinocytes. Alpha-MSH and other bioactive peptides are products of pro-opiomelanocortin (POMC). There is biochemical and genetic evidence demonstrating that UV induction of POMC/MSH in skin is directly controlled by p53. Whereas p53 potently stimulates the POMC promoter in response to UV, the absence of p53 is associated with absence of the UV-tanning response. The same pathway produces b-endorphin, another POMC derivative, which can contributes to sun-seeking behavior. Furthermore, several instances of UV-independent pathologic pigmentation are shown to involve p53 ‘‘mimicking’’ the tanning response. Therefore p53 functions as a sensor/effector for UV pigmentation, which is an everyday environmental exposure. This pathway is activated in numerous conditions of pathologic pigmentation and mimics the tanning response. (Rutao Cui et al. 2007)
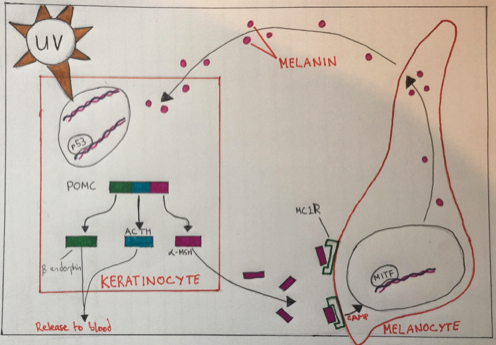
Fig 3.
Figure 3 : The Role of P53 in Skin Pigmentation - In response to UV-induced genotoxic stress, p53 becomes activated in skin keratinocytes and stimulates transcription from the pro-opiomelanocortin (POMC) gene promoter. The POMC pre- cursor polypeptide is then processed into several bioactive products including α-MSH, which, through a paracrine effect on epidermal melanocytes (mediated by the α-MSH receptor MC1R and the melanocytic transcription factor MITF), leads to melanin production and redistribution among skin cells. The release of ACTH (adrenocorticotropic hormone) and the opioid peptide β-endorphin into the blood may relieve in ammation and contribute to sun-seeking behavior. (Moshe Oren and Jiri Bartek)
Molecular aspects of tanning
Tanning is understood as an increase in epidermal melanisation in skin following UV radiation, but can have a further role in a host response that protects against further UV-induced damage. Tanning is mainly seen in humans but has been known to be seen in other mammals such as sharks. (Barbara A. Gilchrest, 2011 ) Melanocytes (MC) sit along the epidermal basal layer, have a minimal role except for constitutive melanin production.
They are usually only activated after sun exposure. (Graeme Walker et al, 2008) Coat color study in animals has been an important factor in the study of genes that control pigmentation in humans. In both humans and animals fully differentiated MCs (Melanocytes) can be divided into two major groups, those in the hair bulb, whose function is mainly to produce pigment for hair color, and epidermal MCs, which supply the epidermis with melanin and are involved in the tanning response. (Graeme Walker et al, 2008) After an UVR exposure to the skin, basal keratinocytes proliferate (resulting in epidermal hyperplasia), to replace apoptotic “sunburn cells” that express p53. (Graeme Walker et al, 2008) UVR may cause pigmentation in two ways by affecting the skin melanocytes.
The first which can be called Intermediate pigment darkening, fades rapidly and is due to oxidation of already existing melanin in the melanocytes. The second, called Delayed tanning, appears a lot later as it increases melanogenesis, allowing the darkened skin to be evident a few days after UVR exposure and may last up to a week. (Graeme Walker et al, 2008) For a long time it has been appreciated that tanning is the skins main response in protection against UV radiation, and against acute and chronic damage. What we would consider a “good tan” at best offers a moderate degree of photoprotection. If we compare a lightly pigmentented epidermis with darkly pigmented epidermis, the darkly pigmented epidermis reduces skin UV damage by a factor of 4(Fig. 4).
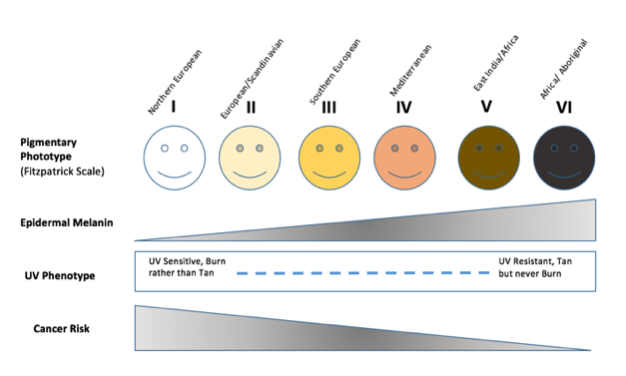
Fig 4.
Figure 4 : Pigmentation Phototype of Humans Demonstrated on the Fitzpatrick Scale
Skin darkening regulated by UV radiation is a biphasic process. The initial darkening is due to redistributioning and molecular changes to already existing melanin pigments within epidermis. The second phase is due to up-regulation in melanin synthesis and its transfer to keratinocytes. (John D’Orazio et al, 2013) This can show that a number of changes occur in the epidermis due to the tanning response including melanogenesis, which is the synthesis of melanin – pigment in hair, eyes and skin color. (Barbara A. Gilchres, 2011) It is known that P53 Factor and KITLG expression are expressed in keratinized cells are exposure to UV. (Graeme Walker et al, 2008) It has been possible to prove that after 24 hours there is a 2-3 fold increase of nucleotide repair protiens, after UV radiation. Also research has discovered that keratinocyte derived gene products are regulated by UV. (Barbara A. Gilchrest, 2011)
Researchers have been unable to discover how P53 regulates KITLG, but they have ideas based on the fact that most paracrine released from keritized cells after UV exposure are regulated by P53, and that KITLG has receptors for P53 allowing regulation. (Graeme Walker et al, 2008) UV has many other effects on the skin, including induction of an immune-tolerant or immunosuppressive state and production of vitamin D by direct conversion of 7-dehydrocholesterol into vitamin D3. (John D’Orazio et al,2013)
Artificial tanning
Over the years the number of artificial tanning methods such as indoor tanning salons have become more popular. In 1980 only 1% of the American population had ever used a sunbed, whereas now a huge 25% have used, or use them regularly (Fig. 5).
Indoor tanning salons are known to be poorly regulated in terms of the strength of UV radiation, they can be up to 10 times stronger than the UV inflicted by the sun, making the tanning beds an authentic carcinogenic instrument. It has been proven that tanning can be addictive, leading to more frequent and intense UV exposure.

Fig 5.
Figure 5 : A Commercially Used Tanning Bed
Lifetime risk of skin melanoma increases by 75%for sunbed users under the age of 35. Since the molecular pathways in the skin that activate UV-induced tanning result from cellular and DNA damage it can result in skin damage and carcinogenesis, it appears this is the same case for tanning salons but appears to be more intense.
For business purposes the industry commercializes the UV health benefits, emphasizing the Vitamin D production, naturally made by the skin through 7-dehydrocholesterol into Vitamin D3, but research has suggested that every day sun exposure as well as Vitamin D containing foods and supplements exceed the required vitamin D levels the body needs. This means extra exposure through tanning salons is unnecessary to prevent diseases such as rickets. Decreasing UV radiation exposure, both naturally from sunlight and artificially from tanning bed use, may be the single best way to reduce incidence of melanoma and other skin cancers. (John D’Orazio et al, 2013)
Skin Cancer relating to P- 53
The ultraviolet (UV) component of sunlight is a carcinogen that everyone is exposed to. Squamous cell carcinomas (SCC) are one of the main types of skin cancer, and begins in the squamous cells in the skin. It is caused by the uncontrolled growth of abnormal cells in the squamous cell layer, which compose most of the skins upper layers, such as the epidermis. SCCs can appear as elevated growths with a central depression, warts and scaly red patches. SCC typically occur in individuals older than 50 ("Squamous Cell Carcinoma - Causes and Risk Factors - SkinCancer.org". www.skincancer.org. Retrieved 25 March 2017.)
SCCs are caused by cumulative UV exposure over the course of a lifetime, whereas basal cell carcinomas (BCC) are caused by cumulative and intense exposure to sunlight, e.g. sunburn.
Douglas et al states that 58% of invasive SCC have mutations in the P53 tumour suppressor gene. Mutations due to direct sunlight absorption have a CC-TT double base change - which is only understood to be induced by UV – or C-T base changes. UV can be identified as the mutagen as it is the only known cause for this mutation. C- A
Two methods have been developed to determine the UV specific p53 gene mutations in UV exposed normal skin. one of them is by detecting the CC-TT double base change in DNA samples using mutant allele specific PCRs and ligase chain reactions.
These double base changes were detected in cultured human skin cells after exposure to UV radiation. Increasing the dosage of UV radiation resulted in an increase of the mutation frequency, the number of times the mutation occurs. In their study, 17 of the 23 samples of normal sun skin from sun exposed sites (74%) from Australian skin cancer patients contained CC to TT double base changes in one or both of codons 245 and 247/248 of the p53 gene. In contrast, only 1 of 20 (5%) samples form non exposed sites had the mutation. Mutations at codons 247/248 occurred more frequently than those at codon 245. Samples taken from non-exposed sites from people who had intermittent exposure to UV had no similar mutations. The results of the study suggested that “specific p53 gene mutations associated with human skin cancer are induced in normal skin by solar UV radiation. To examine the link between sun exposure and UV specific p53 gene mutations in human skin, normal skin biopsies were taken from exposed areas, the shoulder, and non-exposed areas, the buttocks. When the codons of the p53 gene were tested, the biopsy form the exposed area showed mutations in 76% of the samples. Biopsies form the non-exposed buttock area showed mutations in 8.3% of the samples.
Sunlight induced skin cancer is doubling every decade in the united states.
Role of P53 as a Prognostic factor for Cancer
P53 as a Prognostic Factor for the Likely Course of Lung Cancer
Mutations of the p53 gene are implicated in the carcinogenesis of cancers such as lung cancer. P53 can be used to predict the likely course of lung cancer. It is a 53-kD nuclear phosphoprotein, produced by the p53 tumour suppressor gene. It is localized on the human chromosome 17p13. There has been numerous studies done with a small series of patients. The criteria to be eligible for the study is to deal with lung cancer only; to evaluate the correlation between p53 and the survival rate of the patient; to analyse p53( protein, DNA or RNA) in the primary tumour or antibodies in the serum. to assess the prognostic value of p53 abnormalities for the survival of patients presenting with lung cancer.
The results of the study that alteration of the p53 gene in the lung cancer cells is a poor prognostic factor for survival in patients with NSCLC (non small cell lung cancer). The analysis revealed similar features for each NSCLC subgroup and helped to clarify the somewhat inconsistent message of individual studies. This observation is potentially important for prognostic reasons. Identification of independent prognostic factors allowed for the definition of high-risk. (E.steels et al, 2001)
P53 as a Prognostic Factor for Breast Cancer
P53 was also Identified as a prognostic and predictive biomarker for breast cancer. Breast cancer is the most prevalent cancer and the leading cause of cancer death in female worldwide. It also accounts for 25% of all cancer cases and 15% of all cancer deaths in women. Triple-negative breast cancer (TNBC) takes up about 15% of all breast cancers and lacks estrogen receptor and progesterone receptor expression as well as human epidermal growth factor receptor 2 (HER2) amplification. Compared to other subtypes of breast cancer, TNBC is more biologically aggressive and has higher recurrence rate, higher frequency of metastasis and worse survival.
The clinicopathological parameters of this subgroup consist of large tumors size, multiple apoptotic cells, high proliferative index, highly undifferentiated, central necrosis and high positivity of lymph node involvement. P53 plays a vital role in determining cell fate exposed to DNA damage stimuli. Alterations of p53 have been investigated in recent years. (Yunbao Pan et al, 2017) Studies suggest from the research article on the prognostic marker of p53 and Ki-67in triple negative breast cancer that P53 gene is the most frequently mutated tumor suppressor gene in human malignancy, with 30% breast cancers having P53 mutation.
The frequency of P53 mutation in breast cancer relies on molecular subset. With luminal subgroup having the lowest mutation and basal subgroup has highest mutation. The mutation of P53 gene can be a representative of an early event in tumor progress, because it is evident at the in situ phase of cancer growth. P53 mutation probably stimulates cell proliferation and renders aggressive phenotype. The availability of detecting mutant p53 protein on formalin-fixed paraffin-embedded (FFPE) tissue has allowed the retrospective studies of patients with a long follow-up.
In the study done patients were between the age of 28-85. In the 78 cases 50% of these were menopause. The main histological type was lobular, ductal and medullary. 19 had a grade 1 tumor; 89 had a grade 2 tumor; 48 had a grade 3 tumor. Based on tumor staging system, most patients were defined as stage II and stage I. The expression of p53 and Ki-67 in TNBC were detected by IHC. Correlations between p53 positive cases or Ki-67 high index cases and clinicopathological parameters were summarized. The distribution of most clinicopathological parameters is similar in the p53 positive patients, as well as in the patients with Ki-67 high index. However, most patients p53 levels was associated with Ki-67 levels in TNBC. However, we did not observe any association between p53/Ki-67 and histological type, tumor stage, family history nor menopause. (Yunbao Pan et al, 2017)
References
Articles & Journals
- CUI, R., WIDLUND, H. R., FEIGE, E., LIN, J. Y., WILENSKY, D. L., IGRAS, V. E., D'ORAZIO, J., FUNG, C. Y., SCHANBACHER, C. F., GRANTER, S. R. AND FISHER, D. E. Central Role of p53 in the Suntan Response and Pathologic Hyperpigmentation In-text: (Cui et al. 853-864) Your Bibliography: Cui, Rutao et al. "Central Role Of P53 In The Suntan Response And Pathologic Hyperpigmentation". Cell 128.5 (2007): 853-864.
- D'ORAZIO, J., JARRETT, S., AMARO-ORTIZ, A. AND SCOTT, T. UV Radiation and the Skin In-text: (D'Orazio et al. 12222-12248) Your Bibliography: D'Orazio, John et al. "UV Radiation And The Skin". International Journal of Molecular Sciences 14.6 (2013): 12222-12248.
- GILCHREST, B. A. Molecular Aspects of Tanning In-text: (Gilchrest E14-E17) Your Bibliography: Gilchrest, Barbara A. "Molecular Aspects Of Tanning". Journal of Investigative Dermatology 131 (2011): E14-E17.
- GROSSMAN, D. The molecular basis of nonmelanoma skin cancer: new understanding In-text: (Grossman 1263-1270) Your Bibliography: Grossman, D. "The Molecular Basis Of Nonmelanoma Skin Cancer: New Understanding". Archives of Dermatology 133.10 (1997): 1263-1270.
- HUSSEIN, M. R., HAEMEL, A. K. AND WOOD, G. S. p53-related pathways and the molecular pathogenesis of melanoma In-text: (Hussein, Haemel and Wood 93-100) Your Bibliography: Hussein, M R, A K Haemel, and G S Wood. "P53-Related Pathways And The Molecular Pathogenesis Of Melanoma". European Journal of Cancer Prevention 12.2 (2003): 93-100.
MAYA, R., BALASS, M., KIM, S., SHKEDY, D., MARTINEZ LEAL, J. -. F., SHIFMAN, O., MOAS, M., BUSCHMANN, T., RONAI, Z., SHILOH, Y., KASTAN, M. B., KATZIR, E. AND OREN, M. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage In-text: (Maya et al. 1067-1077) Your Bibliography: Maya, Ruth et al. "ATM-Dependent Phosphorylation Of Mdm2 On Serine 395: Role In P53 Activation By DNA Damage". Genes & Development 15.9 (2001): 1067-1077.
- NAKAZAWA, H., ENGLISH, D., RANDELL, P. L., NAKAZAWA, K., MARTEL, N., ARMSTRONG, B. K. AND YAMASAKI, H. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. In-text: (Nakazawa et al. 360-364) Your Bibliography: Nakazawa, H. et al. "UV And Skin Cancer: Specific P53 Gene Mutation In Normal Skin As A Biologically Relevant Exposure Measurement.". Proceedings of the National Academy of Sciences 91.1 (1994): 360-364.
- OREN, M. AND BARTEK, J. The Sunny Side of p53 In-text: (Oren and Bartek 826-828) Your Bibliography: Oren, Moshe, and Jiri Bartek. "The Sunny Side Of P53". Cell 128.5 (2007): 826-828.
- PAN, Y., YUAN, Y., LIU, G. AND WEI, Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients In-text: (Pan et al. e0172324) Your Bibliography: Pan, Yunbao et al. "P53 And Ki-67 As Prognostic Markers In Triple-Negative Breast Cancer Patients". PLOS ONE 12.2 (2017): e0172324. Web. WALKER, G. AND BOX, N. Ribosomal stress, p53 activation and the tanning response In-text: (Walker and Box 649-656) Your Bibliography: Walker, Graeme, and Neil Box. "Ribosomal Stress, P53 Activation And The Tanning Response". Expert Review of Dermatology 3.6 (2008): 649-656.
Websites
https://en.wikipedia.org/wiki/TP53
http://www.bioinformatics.org/p53/introduction.html
https://www.britannica.com/science/melanocyte-stimulating-hormone
Figures
Figure 1 - “P53” By Thomas Splettstoesser https://commons.wikimedia.org/w/index.php?curid=1248114
- Figure 2 -“The Role of P53” –Chène, Patrick. "Figure 2 : Inhibiting The P53–MDM2 Interaction: An Important Target For Cancer Therapy : Nature Reviews Cancer".
- Nature.com. N.p., 2017. Web. 2 May 2017.- (Illustrated by Rosie Ansell)
- Figure 3 -“The Role of P53 in Skin Pigmentation" Oren, Moshe, and Jiri Bartek. "The Sunny Side Of P53". Cell 128.5 (2007): 826-828. Web. (Illustrated by Rohan Madhavan)
- Figure 4 – “Pigmentary Phototype” – D'ORAZIO, J., JARRETT, S., AMARO-ORTIZ, A. AND SCOTT, T. UV Radiation and the Skin In-text: (D'Orazio et al. 12222-12248) Your Bibliography: D'Orazio, John et al. "UV Radiation And The Skin". International Journal of Molecular Sciences 14.6 (2013): 12222-12248. Web.- Illustrated by Rosie Ansell
Figure 5 - A Commercially Used Tanning Bed - By Dmitry G - Cropped from File:Sütiste tee 32 - tanning bed installing.JPG, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=52778889
