|
Size: 424
Comment:
|
Size: 4650
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 4: | Line 4: |
== Introduction to P53 == |
|
| Line 12: | Line 14: |
P53 (TP 53) is a tumor suppressor gene that is considered one of the most frequent targets for gene alterations relating to cancer. (Moshe Oren et al, 2007) It codes for a protein that regulates the cell cycle, functioning as a tumor suppressor. In 1993 P53 was voted molecule of the year by science magazine, and has been described as “the guardian of the genome”, relating to ability to prevent genome mutation. In over half of human tumors we can see the inactivation of P53. Due to many experiments on the biochemistry an physiology of P53 relating to its cancer role, it is now appreciated that its main role is to prevent the emergence of cells with permanently defective genomes, which are very likely to lead to cancer. In normal cells, P53 protein is low, but after DNA damage or other stress such as UV radiation, a trigger response may occur, increasing the number of P53 proteins in the cell. These proteins have three major functions: apoptosis (cell death), DNA repair and growth arrest – stopping the cell cycle and therefore preventing replication of damaged DNA. Apoptosis would be a final result to prevent replication of damaged DNA and avoiding tumor growth. Although high levels of P53 can be considered a good thing when referred to cancer suppression, too much can result in accelerated aging which will be discussed further as an effect of excessive UV radiation. == Role of P-53 and relation to MSH == P53 is a tumour suppressor gene and is located on the seventeenth (17p13) chromosome. It is one of the most frequent targets for genetic alteration in cancer. A lot of human tumours are able to mutationally inactivate p53, removing its role as a tumour suppressor and preventing the emergence of cells with permanently defective genomes, which can often cause cancer. P53’s ability is largely due to it being able to act as a sequence special c transcriptional regulator. (Moshe Oren et al, 2007) It is also a key regulator of cellular responses to geno-toxic stress, and it is stabilised and activated after DNA damage. The rapid activation of p53 by ionising radiation mostly dependents on the ATM kinase. P53 is phosphorylated by ATM shortly after DNA damage, resulting in enhanced stability and activity of p53. The Mdm2 oncoprotein is a major negative regulator of P53, triggering its degradation by ubiquitin system. In response to ionising radiation and radio-mimetic drugs, Mdm2 undergoes rapid ATM-dependent phosphorylation before to p53 accumulation. This decreases its reactivity with the 2A10 monoclonal antibody. Phage display analysis is a laboratory technique, for the study of proteins and protein-DNA that use bacteriophages to connect proteins with the genetic information. From this analysis a consensus 2A10 recognition sequence was identified, possessing the core design DYS. Unexpectedly, this design appears twice within the human Mdm2 molecule, at positions corresponding to residues 258–260 and 393–395. Serine 395, residing within the carboxy-terminal 2A10 epitope, is the major target on Mdm2 for phosphorylation by ATM in vitro. (Ruth Maya et al, 2001) Alpha-Msh is a a-melanocyte- stimulating hormone secreted by keratinocytes. Alpha-MSH and other bioactive peptides are products of pro-opiomelanocortin (POMC). There is biochemical and genetic evidence demonstrating that UV induction of POMC/MSH in skin is directly controlled by p53. Whereas p53 potently stimulates the POMC promoter in response to UV, the absence of p53 is associated with absence of the UV-tanning response. The same pathway produces b-endorphin, another POMC derivative, which can contributes to sun-seeking behavior. Furthermore, several instances of UV-independent pathologic pigmentation are shown to involve p53 ‘‘mimicking’’ the tanning response. Therefore p53 functions as a sensor/effector for UV pigmentation, which is an everyday environmental exposure. This pathway is activated in numerous conditions of pathologic pigmentation and mimics the tanning response. (Rutao Cui et al. 2007) |
P53: The Key to Sunbathing (The Regulation of MSH)
Introduction to P53
This essay discusses the effects of P53, its relation to sunbathing and the tanning response.
Additionally, we will be discussing the P53’s relation to cancer and its prognostic factor and
the role of MSH.
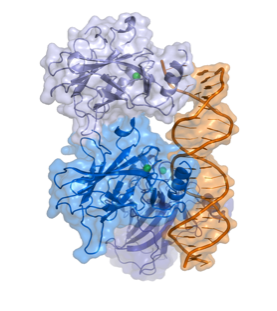
Fig 1.
The Structure of P53 (Splettstoesser)
P53 (TP 53) is a tumor suppressor gene that is considered one of the most frequent targets
for gene alterations relating to cancer. (Moshe Oren et al, 2007) It codes for a protein that
regulates the cell cycle, functioning as a tumor suppressor. In 1993 P53 was voted molecule
of the year by science magazine, and has been described as “the guardian of the genome”,
relating to ability to prevent genome mutation.
In over half of human tumors we can see the inactivation of P53. Due to many experiments
on the biochemistry an physiology of P53 relating to its cancer role, it is now appreciated
that its main role is to prevent the emergence of cells with permanently defective genomes,
which are very likely to lead to cancer.
In normal cells, P53 protein is low, but after DNA damage or other stress such as UV
radiation, a trigger response may occur, increasing the number of P53 proteins in the cell.
These proteins have three major functions: apoptosis (cell death), DNA repair and growth
arrest – stopping the cell cycle and therefore preventing replication of damaged DNA.
Apoptosis would be a final result to prevent replication of damaged DNA and avoiding
tumor growth. Although high levels of P53 can be considered a good thing when referred to
cancer suppression, too much can result in accelerated aging which will be discussed further
as an effect of excessive UV radiation.
Role of P-53 and relation to MSH
P53 is a tumour suppressor gene and is located on the seventeenth (17p13) chromosome. It
is one of the most frequent targets for genetic alteration in cancer. A lot of human tumours
are able to mutationally inactivate p53, removing its role as a tumour suppressor and
preventing the emergence of cells with permanently defective genomes, which can often
cause cancer. P53’s ability is largely due to it being able to act as a sequence special c
transcriptional regulator. (Moshe Oren et al, 2007)
It is also a key regulator of cellular responses to geno-toxic stress, and it is stabilised and
activated after DNA damage. The rapid activation of p53 by ionising radiation mostly
dependents on the ATM kinase. P53 is phosphorylated by ATM shortly after DNA damage,
resulting in enhanced stability and activity of p53.
The Mdm2 oncoprotein is a major negative regulator of P53, triggering its degradation by
ubiquitin system. In response to ionising radiation and radio-mimetic drugs, Mdm2
undergoes rapid ATM-dependent phosphorylation before to p53 accumulation. This
decreases its reactivity with the 2A10 monoclonal antibody. Phage display analysis is a
laboratory technique, for the study of proteins and protein-DNA that use bacteriophages to
connect proteins with the genetic information. From this analysis a consensus 2A10
recognition sequence was identified, possessing the core design DYS. Unexpectedly, this
design appears twice within the human Mdm2 molecule, at positions corresponding to
residues 258–260 and 393–395. Serine 395, residing within the carboxy-terminal 2A10
epitope, is the major target on Mdm2 for phosphorylation by ATM in vitro. (Ruth Maya et al,
2001)
Alpha-Msh is a a-melanocyte- stimulating hormone secreted by keratinocytes. Alpha-MSH
and other bioactive peptides are products of pro-opiomelanocortin (POMC). There is
biochemical and genetic evidence demonstrating that UV induction of POMC/MSH in skin is
directly controlled by p53. Whereas p53 potently stimulates the POMC promoter in
response to UV, the absence of p53 is associated with absence of the UV-tanning response.
The same pathway produces b-endorphin, another POMC derivative, which can contributes
to sun-seeking behavior. Furthermore, several instances of UV-independent pathologic
pigmentation are shown to involve p53 ‘‘mimicking’’ the tanning response. Therefore p53
functions as a sensor/effector for UV pigmentation, which is an everyday
environmental exposure. This pathway is activated in numerous conditions of
pathologic pigmentation and mimics the tanning response. (Rutao Cui et al. 2007)
