Itt írjon a(z) ParkinsonTreatment-ról/ről
Treatment of Parkinson’s disease
What is Parkinson’s disease?
Parkinson’s disease is a condition that affects dopaminergic neurotransmission. Dopamine is essential for controlling movement, cognition, and emotion in the Central Nervous System. Parkinson’s Disease damages dopamine neurotransmission, leading to impaired movement in the form of rest tremor, rigidity, and slowness of movement (bradykinesia) (Schuepbach et al, 2013). The condition can also cause non-motor symptoms, including cognitive impairment, dementia, psychosis, apathy, tiredness, sleep disorders, and disturbances of mood (Goldman and Holden, 2014).
What causes it?
Parkinson’s disease is caused by the progressive loss of dopaminergic neurons or the disturbance of dopamine signalling (Lu et al, 2017).
Treatment
Unfortunately there is currently no real cure for Parkinson’s disease. Current treatment for Parkinson’s disease involves managing the symptoms of the condition as it progresses (Aviles-Olmos et al, 2013). The main goal is to give patients the highest quality of life possible by slowing down the advance of disease and treating and minimising the symptoms.
Pharmaceutical treatments
Levodopa (also known as L-DOPA) is an amino acid that is the precursor of many important neurotransmitters, such as dopamine, epinephrine, and norepinephrine (together they are known as catecholamines). It also mediates the release of neurotrophic factors from the Central Nervous System. Levodopa can be synthetically produced as a purified psychoactive drug, and has been used for many years in treating Parkinson’s disease. By taking Levodopa medication, the patient can make up for the lost dopaminergic neurons, thus allowing for more normal dopamine signalling (Brichta et al, 2013).
A study carried out between 2000 and 2009 showed that levodopa provides small but continuing benefits, as compared to levodopa-sparing therapy (the latter is a treatment in which the use of levodopa is avoided or limited for as long as is practically possible). It is thought that levodopa provides better short-term control of the motor symptoms in patients with newly diagnosed Parkinson’s disease. It also seems to cause fewer side-effects than dopamine agonists or Monoamine Oxidase Type B Inhibitors (MAOBI) (UK PD MED Collaborative Group, 2014)
Unfortunately, according to Lu et al (2017), chronic use of Levodopa leads to L-DOPA-Induced Dyskinesia (LID) (involuntary muscle movements such as twitching or tics); however, if one stops taking Levodopa, dyskinesia may also evolve (Mizuno et al, 2016). Lu et al (2017) mention that the negative effects of Levodopa can be reduced if the drug is used in combination with Dopa agonists or if dopa agonists are used in monotherapy.
Monoamine Oxidase Type B Inhibitors (MAOBI) have been used in levodopa sparing therapy. They were found to be a treatment that is at least as effective as dopa agonists (UK PD MED Collaborative Group, 2014)
There has also been research in using Exanatide, often given to patients with Type 2 Diabetes, to treat Parkinson’s disease. Exenatide has been shown to have some neuroprotective and neurorestorative properties, and it might be a more cost effective treatment. In a recent study, patients with moderate Parkinson’s disease were given subcutaneous injections of Exenatide for about 12 months. The results of the study showed that the drug was well-tolerated by these patients, although weight loss was common. The results seem promising, but further research is needed (Aviles-Olmos et al, 2013).
For a quick summary of the previous mentioned drugs and their effects, see table 1.
|
Advantages |
Disadvantages |
Levodopa |
Increases Dopamine signalling. Provides better motor function control |
Increases dyskinesia |
Exanatide |
Neuro protective, Neuro restorative |
New method, further testing is needed |
Dopamine Agonists |
Decreases dyskinesia |
Side effects |
MAOBI |
Decreases dyskinesia |
Side effects |
Mitochondria-targeted Antioxidants
It has been suggested that mitochondrial dysfunction and oxidative stress have an important role in the dopaminergic neurodegeneration of Parkinson's disease. Thus, therapies that target and improve their function mitochondria could help to prevent or treat Parkinson’s disease. A class of compounds called Mitochondria-targeted antioxidants has been developed. They are created by conjugating lipophilic triphenylphosphonium cation to an antioxidant moiety. Studies seem promising, but further research is needed, as the pharmacological effects on these drugs are not entirely known (Huajun et al, 2013).
Deep brain stimulation
If pharmaceutical treatment proves ineffective, another option is neurostimulation. Deep brain stimulation uses electrodes to target certain parts of the brain, brain surgery is required to place an electrode on the targeted area. A wire will go from the electrode, under the skin, to the neurostimulator placed under the skin around the area of the collarbone. This neurostimulator sends a stimulus with a set frequency to the electrode and stimulates the targeted area in the brain (parkinson.org).
Depending on where the electrode is placed in the brain, it will affect different symptoms (see Figure 1). The electrode placement is decided individually for each person undergoing the surgery, according to the advancement of the individual's disease. The most commonly used and studied areas for electrode placement include the subthalamic nucleus (STN), globus pallidus internus (GPi), thalamic ventralis intermedius (Vim) and the pedunculopontine nucleus (PPN). There are long-term studies (10 years) available for STN DBS, whereas only medium-term studies (5 years) are available for GPi and Vim DBS. For PPN DBS, only short-term studies (2 years) are available. DBS can be performed with or without addition of dopamine replacement treatment (DRT), and different levels of DRT reduction depending on the patients need (Fasano et al, 2012).
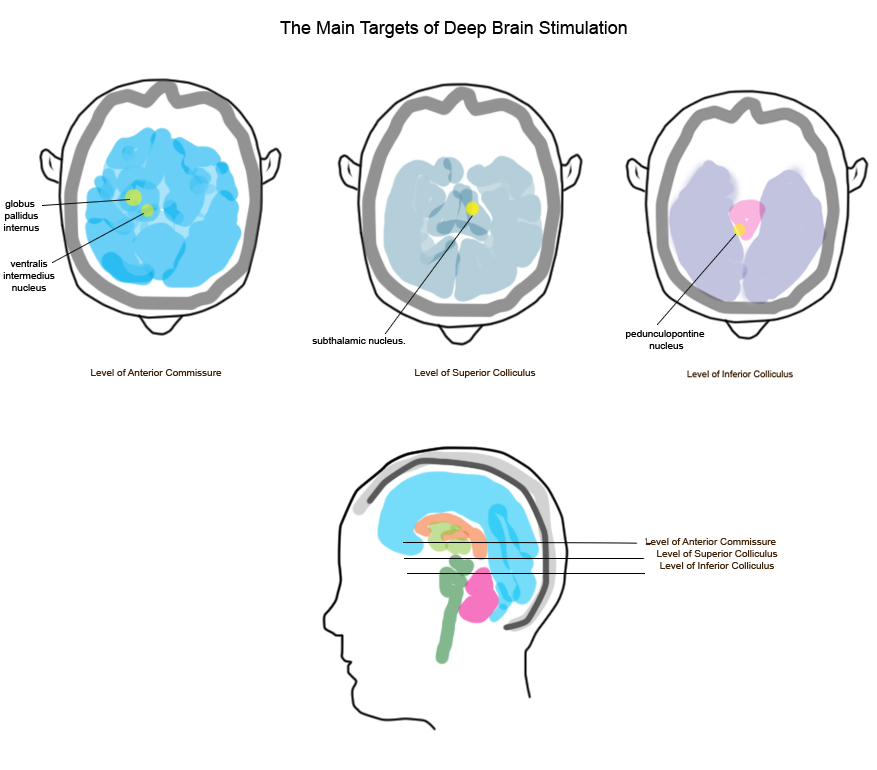
Fig. 1. The Main Targets of Deep Brain Stimulation
The different placements will have different effects and side-effects, as shown in Table 2.
Electrode Placement |
Improvement |
Side effects |
Subthalamic nucleus (STN) |
- Rigidity and bradykinesia (increased improvement with DRT) - Tremors - Gait and balance (short-term) - Motor fluctuations Dyskinesia (Reduction of DRT) - Impulse control disorders (ICD) - Dopamine dysregulation syndrome (DDS) - Depression (due to improved motor symptoms) - Psychosis -Dysautonomia - Sleep |
-Speech impairments (long-term) -Induce ICDs in patients with high DRT doses -Worsen/induce punding -Apathy worsening -Mood disorders |
Globus pallidus internus (GPi) |
- Rigidity and bradykinesia - Tremors - Gait and balance (short-term) - Dyskinesia |
- Delayed speech and dysarthria - Apathy worsening |
Thalamic ventralis intermedius nucleus (Vim) |
- Tremors |
- |
Pedunculopontine nucleus (PPN) |
Gait and balance - Reaction time for executive function and working memory - Delayed recall and verbal fluency |
|
|
Both STN and GPi DBS significantly reduce bradykinesia and rigidity, while thalamic stimulation has no effect on these symptom. The effect of PPN stimulation is still disputed. STN DBS has a greater effect on bradykinesia than GPi DBS, (70-80% compared to 30-40% according to retrospective comparisons) (Fasano et al, 2012).
Tremors
Vim DBS is considered the best option for tremor symptoms, though SNT and GPi DBS also improve tremors. Vim DBS is especially suitable for older patients when other targets are not practicable. There have also been suggestions that the centremedian/parafascicular (CM/Pf) thalamic complex is a successful target for relieving tremor symptoms. So far only long-term efficacy of STN, GPi and thalamic DBS has been reported with regard to controlling tremors (Fasano et al, 2012).
Gait and balance
Gait and balance problems usually occur in late stages of Parkinson’s disease, approximately 10-15 years after onset. Studies show that during the first year after STN implantation, stimulation improved postural instability gait difficulty (PIGD) complex. Addition of drug treatment showed an even greater improvement in the short term; however, results are highly individual, and there have been several cases of poor or no gait improvement after STN implantation, even in the short run. In the long run, axial motor features decline despite the DBS treatment, and gait problems can arise even after stimulation in some cases. Studies show that both STN and GPi DBS will initially improve PIGD, but usually 2 years after surgery, values gradually returns to pre-surgery values in STN implantation (but not in GPi implantations). PPN DBS has been suggested for patients with severe axial symptoms that are unresponsive to drug treatments. Initial reports show an improvement of gait with low frequency stimulation, but a worsening with higher frequencies (Fasano et al, 2012).
Speech
STN DBS shows significant improvements in speech intelligibility, phonation, or articulation. This seems to be only temporarily, as the effects usually weaken over time. A retrospective finding is that speech worsens after STN implantation in as many as 90% of patients after 8 years. There is also delayed speech worsening after 5-6 years in patients with GPi implants, as well as stimulation-induced dysarthria (Fasano et al, 2012).
Motor fluctuations and dyskinesia
SNT DBS gives beneficial reduction in motor fluctuations that will last for several years after the surgery. STN DBS does not have an antidyskinetic effect, but because of the reduction of DRT there has been reported reduction of dyskinesia after SNT implantation. GPi DBS, on the other hand, has a direct and acute antidyskinetic effect when stimulation is delivered though the ventral regions (Fasano et al, 2012).
Non Motor Symptoms
Cognition
In STN DBS there have been consistently reports of postoperative decline on phonological and semantic verbal fluency tasks. There have also been reports of decline in tasks of episodic memory, executive function, and abstract reasoning. The postoperative reduction of DRT might be the reason for decline in verbal fluency. SNT DBS is deemed safe from a cognitive standpoint if a strict inclusion criteria are used. GPi DBS has low cognitive effects, and there has only been reported mild declines in semantic verbal fluency. Long-term studies conclude that there are fewer cognitive adverse events in GPi DBS than SNT DBS. PPN stimulation generally reduces reaction time in executive function tests and working memory, as well as improved performance on delayed recall and verbal fluency (Fasano et al, 2012).
Impulse control disorders
STN DBS can improve or even eliminate pre-existing impulse control disorders (ICD). This might be because of DRT reduction after implantation; however, SNT DBS can also mimic the action of DRT and induce ICDs, especially in patients taking high doses of DRT (Fasano et al, 2012).
Dopamine disregulation syndrome and punding
Patients with dopamine dysregulation syndrome symptoms improved greatly with SNT stimulation. Punding, which is triggered by DRT, is a stereotypical behaviour characterized by intense fascination with complex, excessive, repetitive activities. This behaviours might worsen or even arise after SNT implantation, even if DRT is reduced (Fasano et al, 2012).
Apathy
Studies report worsening of apathy after SNT and GPi DBS, but this can be reversed in some cases (Fasano et al, 2012).
Mood disorders
STN stimulation can induce postoperative mood disorders (depression or mania). Due to the improvement of motor symptoms, depression may actually be reduced. Mania may be reduced or resolved with the readjustment of the stimulation settings, or by switching to another stimulation target (Fasano et al, 2012).
Psychosis
Studies show various results, but in most cases psychosis disappear after STN DBS with reduction of DRT. In other stimulation targets, there have been few reports of psychotic adverse events (Fasano et al, 2012).
Autonomic dysfunction
STN DBS with DRT reduction can improve dysautonomia. This have significant importance for bowel functions (Fasano et al, 2012).
Sleep
There has also been reported improvement of sleep and increased sleep time after STN implantation (Fasano et al, 2012).
MR-guided focused ultrasound treatment
There have also been recent developments in ultrasound therapy especially in treatment of tremor disorders. In deep-brain ultrasound therapy, MR-guided focused ultrasound (MRgFUS) is used to destroy targeted brain tissue, and an ablative lesion is created. The process requires that the patient’s skull is fixed with a stereotactic frame; this is because movement of the patient will cause the incorrect targeting for the lesioning. The lesions are made with a focused ultrasound transducer with the help of Magnetic Resonance Imaging (MRI) to find the target area. The tissue is heated up gradually while the patient is monitored in a conscious state both by MRI and personal monitoring. The sonication is applied until either tremor disappears or there is an adverse effect (for example, paraesthesias). Studies report that the tissue temperature needed for beneficial tremor improvement has a threshold of 50°C. The monitoring of the patients includes tasks as drawing or writing to see when the complete or almost complete cessation of the tremors appear, while the patient is still under the MR-guided focused ultrasound apparatus. Check-ups of patients report over 80% reduction in tremor still 3 months after therapy. Paraesthesia may occur because of the treatment, in some cases is resoles over time and in others it is persistent, and in most cases it is free of adverse events. There is, however, still need for larger trials of this treatment in order to deem it completely safe (Lipsman et al, 2013).
MRgFUS’s effects have also been tested when it comes to other symptoms of Parkinson’s disease with success. The accuracy of focused ultrasound has also been reported to be better compared with methods requiring brain penetration (Magara et al,2014).
Another significant feature with MRgFUS is that invasive surgery is no longer needed compared with DBS. The lack of skin incisions and opening of the skull this reduce the risks associated with open neurological surgery, such as intracerebral haemorrhage and infections (Lipsman et al, 2013).
To conclude, although DBS treatment shows many significant improvement for people suffering from Parkinson’s disease, results are individual, so not all patients will experience the same results. In many cases, the improvements are not long lasting and will gradually cease over time, some symptoms more than others. It is also important to consider the side-effects, which include a high risk of speech impairment and cognitive problems (such as memory loss). The risks associated with an invasive procedure like DBS, such as infections and haemorrhage, are with consideration. Fortuitously, trials on new stimulation targets like PPN or CM/Pf are being researched. MRgFUS, another treatment being researched, will hopefully become a good surgical treatment choice for Parkinsonians in the future.
Stem cell treatment/Fetal tissue transplants
Stem cell technology has been researched as a therapy for Parkinson’s disease. Foetal ventral mesencephalic cells have been transplanted into brains affected by the condition. The results have provided proof of concept that replacing the damaged cells can be beneficial for some patients, and greatly improves their motor skills. This form of treatment is controversial, as some question whether using foetal tissue in this way is ethical. There are also practical concerns about this therapy (González et al, 2015)
Pluripotent cells
The theory behind this treatment is that Human Embryonic Stem Cells, obtained from the inner cell mass of blastocysts, have the potential to transform into any of the specialised cells of the three germ layers. It is thought that these cells could be “programmed” to transform into particular cells; in the case of Parkinson’s disease, cells to replace the damaged dopaminergic receptors (González et al, 2015)
Human Induced Pluripotent Stem Cells are similar to Human Embryonic Stem Cells, but can be produced from treated adult human fibroblasts. There is a somewhat larger risk with Human Induced Pluripotent Stem Cells. Current methods for implantation include use of retroviral or lentiviral vectors, which increases the risk of oncogenic transformation and insertional mutagenesis (González et al, 2015).
Multi-potent cells
Human Neural Stem Cells are multi-potent cells capable of self-renewal, and can differentiate into the various types of neural cells. Unfortunately, these cells frequently do not survive being grafted into the brain, and repeated passages in culture leads to these cells losing these properties (González et al, 2015).
Human Mesenchymal Stem Cells are another source of multi-potent tissue. They can be found in many sources, such as adult bone marrow, umbilical cord, and dental tissues, to name a few. It is thought that the use of these cells in stem cell therapy could avoid the need for immunosuppressant drugs, as the patient’s own cells could be used. The use of these cells is also less controversial than the use of foetal tissue. The disadvantage of these cells is that they lack the strong pluripotent capacity of pluripotent cells (González et al, 2015).
According to González et al (2015), the effectiveness, safety, and methodology of this treatment all need further improvement and study. Currently there seems to be a lack of standard surgical methods, as well as a lack of a standard method of evaluating grafts. The best type of cell for this treatment must also be identified, and more research on the effects of inflammatory and immunological processes concerning Parkinson’s disease is needed.
Treatment for cognitive impairment
Although Parkinson’s disease is often associated with motor problems, cognitive impairment can also result from this condition. Dementia, psychosis, depression, anxiety, apathy, impulse control disorders, fatigue, and sleep disturbances are all possible non-motor symptoms. The problem is only magnified by the fact that many drugs used to treat psychosis have dopamine-blocking qualities, worsening the motor symptoms of the disease (Goldman and Holden, 2014).
Dementia
Currently, no drugs are available to improve or prevent cognitive decline in Parkinson’s disease. Cognitive changes and/or impairment in Parkinson’s disease are connected to changes in dopaminergic, cholinergic, and possibly noradrenergic and glutamatergic activity. Cholinesterase inhibitors have been used on Parkinson’s patients suffering from dementia and had positive effects on cognitive function and behaviour. Rivastigmine (a cholinergic agent) has been shown to significantly improve cognition, attention, and neuropsychiatric symptoms; it also makes daily living easier for patients.
Memantine (a partial N-Methyl-D-aspartate-receptor antagonist) has been shown to improve cognition in Parkinson’s patients, but results are thus far inconsistent, though it has been shown that the drug may improve sleep behaviour disturbances and quality of life. Levodopa, used to counter the depletion of dopamine, can help to improve cognitive function to a degree. It can be useful in improving memory, but has little to no effect on “attentional set-shifting, associative learning, and verbal, pattern, and spatial recognition memories” (Svenningsson et al, 2012).
Psychosis can be treated with clozapine, and nortriptyline and pramipexole can treat depression in Parkinson’s patients, but the latter two may not be effective treatments for dementia (Svenningsson et al, 2012).
Psychosis
Psychosis is surprisingly common in Parkinson’s disease patients. It takes many forms, ranging from mild phenomena; such as sensing a presence or visual illusions; to severe symptoms including hallucinations and delusions. Visual hallucinations are most common, and occur during periods of decreased environmental stimulation, often featuring fleeting glimpses of small children or animals. The patient is generally aware that their hallucination is not real, but as the disease progresses they may lose this insight (Goldman and Holden, 2014).
Certain medications for Parkinson’s disease treatment, including tricyclic antidepressants, anticholinergics, benzodiazepines, and opioids; may contribute to psychosis. Where possible, the medication should be reduced or discontinued. Unfortunately, as the mental symptoms improve, the motor symptoms may worsen. Alternatively, antipsychotic medication can be given in addition to the other drugs. This can be problematic, as antipsychotic medications that block dopamine receptors may worsen motor symptoms. Neuroleptic sensitivity is another potential complication. This is a severe psychomotor reaction which resembles neuroleptic malignant syndrome (Goldman and Holden, 2014).
Currently Clozapine and Quetiapine are frequently used to treat psychosis in Parkinson’s patients. Clozapine effectively treats psychosis without worsening motor symptoms, as it selectively binds to the Dopamine 1 mesolimbic receptors and not to the Dopamine 2 receptors. The main risk of this drug is a small possibility of agranulocytosis. It also has a few side effects such as drowsiness, orthostatic hypotension or light-headedness, and increased drooling (Goldman and Holden, 2014).
Quetiapine, an atypical dibenzothiazepine, has a similar structure to Clozapine and works in a similar way; however, there is less data available to support its effectiveness. Its main advantage is that patients do not require the blood monitoring that is necessary when taking Clozapine. It is easy to use in clinical settings, and only relatively low doses are needed for effective treatment, but it can cause side effects such as orthostatic hypotension and sedation (Goldman and Holden, 2014).
Pimavanserin is being investigated as a treatment option for psychosis. This drug is an inverse agonist of 5 HT2a receptors, and is useful for Parkinson’s disease patients because it avoids dopamine blockade, and does not produce motor side effects (based on trial results) (Goldman and Holden, 2014).
Choleinesterase inhibitors are also being researched as a treatment. Rivastigmine and donepezil, both examples of the above drug group, could be useful in treating mild to moderate psychosis. Rivastigmine seems more consistently effective in the treatment of psychosis appears to significantly reduce hallucinations (Goldman and Holden, 2014).
Any other antipsychotics are not suitable for treatment of psychosis in Pakrinson’s disease as they lead to dopamine blockade (Goldman and Holden, 2014).
Apathy
Apathy is “a state of decreased motivation that manifests as a decrease in goal-directed behaviours . . . characterised by reduced interests or emotions that cannot be attributed to diminished level of consciousness, cognitive impairment, or emotional distress” (Pagonabarraga et al, 2015).
In Parkinson’s disease, apathy can be caused by the dysfunction of separate but related neural circuits and subdomains. There are also tests available to differentiate apathy from depression, and what type of apathy is affecting a patient. The integrity of cortical and subcortical structures linking the medial and lateral prefrontal cortex with the limbic system is essential in order to prevent apathy in Parkinson’s patients (Pagonabarraga et al, 2015). Reward deficiency syndrome, in which a patient’s emotions are “blunted” and they lose motivation, can be treated using dopamine receptor agonists, bupropion, and methylphenidate (Pagonabarraga et al, 2015).
Emotional distress can also lead to apathy, with increased activity in the subgenual cingulate cortex and decreased activity in the dorsolateral prefrontal cortex and dorsal anterior cingulate cortex. Antidepressants, such as Tricyclic Antidepressants, can be used to treat this form of apathy (Pagonabarraga et al, 2015).
Executive dysfunction is consistently correlated with apathy in neurodegenerative diseases, leading to cognitive inertia. The lateral prefrontal cortex–lateral caudate and putamen–anterior cingulate cortex are both affected. Acetylcholinesterase inhibitors can be used as a treatment (Pagonabarraga et al, 2015).
Apathy may also be the result of auto-activation deficits, such as akinesia. A person suffering from these deficits is lacking in self-initiated behaviours without external stimulation, and as such are is unable to engage with the world around them. The substantia nigra is affected in various parts of the brain, such as the supplementary motor area, anterior cingulate cortex). Dopamine receptor agonists can be used to treat this form of apathy (Pagonabarraga et al, 2015).
Alternative treatments
Although complementary therapies are generally not as effective as the above mentioned treatments, they can be used to enhance the patients’ quality of life. Exercises such as Tai Chi can be used to improve patients’ balance, which is useful for those with mild to moderate Parkinson’s disease (Li et al, 2012).
Exercise can be helpful in preventing Parkinson’s Disease, and can help to reduce the progression of the disease. It is suggested that frequent, even mild exercise such as walking can help to reduce the progression of mild cognitive impairment to dementia (Uitti, RJ, 2012). Physical activity can slow the deterioration of motor functions, and resistance-based exercises can be useful in improving balance and strength (provided that the correct equipment and safety protocols are used) (Li et al, 2012).
The martial art Tai-Chi can also be beneficial for Parkinon’s Disease patients. A 2012 study showed that participants (Parkinson’s disease patients with mild to moderate forms of the condition) who did Tai-Chi in 60 minute sessions, twice a week for 24 weeks, had improved balance. They were less likely to fall, and had ’improved functional capacity” (Li et al, 2012).
References
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; Limousin, P.; Foltynie, F. (2017): Exenatide and the treatment of patients with Parkinson’s disease. : The Journal of Clinical Investigations 123: (6) 2730–2736
- Brichta, L.; Greengard, P.; Flajolet, M. (2013): Advances in the pharmacological treatment of Parkinson’s disease: targeting neurotransmitter systems. Trends in Neurosciences 36: (9) 343–554
- Fasano, A.; Daniele, A.; Albanese, A.(2012): Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neural 11: 429-442
- Goldman J.; Holdman S.; (2014): Treatment of Psychosis and Dementia in Parkinson’s Disease. Current Treat Options Neurology 16: (281) 1-18
González, C.; Bonilla, Flores, A.; Cano, E.; Liste, I. (2015): An Update on Human Stem Cell-based Therapy in Parkinson's Disease. Current Stem Cell Research & Therapy: 10, 1-8
- Huajun J.; Arthi K.; Anamitra G.; Vellareddy A.; Balaraman K.; Anumantha G. K.; (2014): Mitochondria-targeted antioxidants for treatment of Parkinson's disease: Preclinical and clinical outcomes. Biochimica et Biophysica Acta 1842: 1282–1294
- Li, F.; Harmer, P.; Fitzgerald, K.; Eckstrom, E.; Stock, R.; Galver, J.; Maddalozzo, G.; Batya, S.; (2012): Tai Chi and Postural Stability in Patients with Parkinson’s Disease. New England Journal of Medicine 366:511-9.
- Lipsman, N.; Schwartz, M.L.; Huang, Y.; Lee, L.; Sankar, T.; Chapman, M.; Hynynen, K.; Lozano, A.M.(2013): MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. The Lancet Neurology 12: (5) 462-468
- Lu, J.; Li, X.; Wang, Q.; Pei. G.; (2017): Dopamine D2 receptor and β-arrestin 2 mediate Amyloid-β elevation induced by antiparkinson's disease drugs, levodopa and piribedil, in neuronal cells. PLoS ONE 12: (3) 1-18
- Magara, A.; Bühler, R.; Moser, D.; Kowalski, M.; Pourtehrani, P.; Jeanmonod, D.(2014): First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. Journal of Therapeutic Ultrasound 2:11
- Mizuno, Y.; Shimoda, S.; Origasa, H.; (2016): Long-term treatment of Parkinson’s disease with levodopa and other adjunctive drugs. J Neural Transm 1-9
- Pagonabarraga J.; Kulisevsky J; Strafella A P; Krack P. (2015): Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurology 2015; 14: 518–531
- Schuepbach, W.M.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Hälbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; Falk, D.; Mehdorn, M.; Paschen, S.; Maarouf, M.; Barbe, M.T.; Fink, G.R.; Kupsch, A.; Gruber, D.; Schneider, G.-H.; Seigneuret, E.; Kistner, A.; Chaynes, P.; Ory-Magne, F.; Brefel Courbon, C.; Vesper, J.; Schnitzler, A.; Wojtecki, L.; Houeto, J.-L.; Bataille, B.; Maltête, D.; Damier, P.; Raoul, S.; Sixel-Doering, F.; Hellwig, D.; Gharabaghi, A.; Krüger, R.; Pinsker, M.O.; Amtage, F.; Régis, J.-M.; Witjas, T.; Thobois, S.; Mertens, P.; Kloss, M.; Hartmann, A.; Oertel, W.H.; Post, B.; Speelman, H.; Agid, Y.; Schade-Brittinger, C.; Deuschl, G. (2013): Neurostimulation for Parkinson’s Disease with Early Motor Complications. New England Journal of Medicine 368: 610-22
- Svenningsson P.; Westman E.; Ballard C.; Aarsland D. (2012): Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurology; 11: 697–707
- Uitti R; (2012): Treatment of Parkinson’s disease: focus on quality of life issues. Parkinsonism and Related Disorders 18S1 (2012) 34–36
- UK PD MED Collaborative Group (2014): Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet 384: 1196–205
Figures
(All figures and pictures are prepared (redrawn) by students)
- Figure 1: The Main Targets of Deep Brain Stimulation. Based on Fasano, A. et al (2012)
Tables
(All tables are prepared by students)
- Table 1: Comparison of different drugs. Based on Brichta et al (2013), UK PD MED Collaborative Group (2014), Mizuno et al (2016), Lu et al (2017), and Aviles-Olmos et al (2013)
- Table 2: Comparison of electrode placement. Based on Fasano, A. et al (2012)
