Itt írjon a(z) Pseudopregnancy-ról/ről
Canine pseudopregnancy
Tamara Abela, Neil John Cutajar and Patricia Farrugia
Contents
Introduction
Canine pseudopregnancy (PSPG), also known as pseudocyesis, nervous lactation, copycat pregnancy or phantom pregnancy, is a normal self-limiting physiological syndrome that occurs in female dogs in their late diestrual phase. Signs of pregnancy will be shown in the postpartum period when in reality there is no such pregnancy (Feldman and Nelson 1996; Gobello et al, 2001). Subsequently, behavioural and physical changes will occur. These may be covert, i.e., without any clinical signs, or overt, i.e., with clinical signs, and observed between 6-8 weeks after estrus (Gobello et al, 2002).
Although its exact prevalence is not known, it has been estimated that its incidence rate is as high as 50-75% in certain breeds (Johnston, 1980). Voith (1980) and Jochle (1987) shared the opinion through their work that PSPG evolved through the years to the present day because of the need for non-mated wolves to lactate and feed other female wolves’ litters. This is called alloparental care in mammal species that live in groups, where submissive individuals care for the dominants’ offspring without reproducing themselves (Paul et al, 2014).
The purpose of this essay is to present the most relevant aspects of the physiology, clinical signs, diagnosis, treatment and prevention of clinical PSPG in canines while also highlighting information regarding this syndrome in humans and goats.
a) Pseudopregnancy in goats
PSPG is a pathological condition that causes temporary infertility in goats (Souza et al, 2013).
It is one of the major causes of anestrus, i.e, the absence of the expression of estrus, in dairy goats. Thus leading to decreased reproductive activity (Taverne, 2020).
b) Pseudopregnancy in humans
PSPG in humans is characterized as a rare disorder with somatic features seen in non-psychotic women. In addition, PSPG is when a woman believes that she is pregnant due to many symptoms which resemble an actual pregnancy but there is no foetus actually present (Tarín et al, 2013).
Most of the time this syndrome is observed in women who desire to become pregnant mainly due to infertility (Upadhyay, 2008).
The canine estrous cycle
A good understanding of the canine estrous cycle and its endocrinology is needed to fully grasp the concept of PSPG and the hormonal changes that cause it. The bitch is said to be monoestrous and has the onset of the first estrus between 6-10 months of age and goes through estrus every 6 months thereafter (Gobello et al, 2001; Concannon, 2011).
The anestrus phase is marked by ovarian inactivity, endometrial repair and uterine involution and lasts about 6 months. Its termination is marked by the secretion of pituitary gonadotropins, follicle-stimulating hormone (FSH), luteinizing hormone (LH) induced by gonadotropin-releasing hormone (GnRH). The release of LH results in proestrus folliculogenesis (Blendinger, 2007).
This phase is then followed by proestrus, a phase averaging about 9 days where the vulva becomes enlarged and serosanguineous discharge can be seen. A prominent hormonal change of this phase is the LH peak which causes estrogen to decrease and subsequently increases progesterone steadily (Concannon et al, 1977). These changes result in the luteal phase of the ovarian cycle and therefore estrus is reached.
Estrus lasts about 9 days on average and is the part of the cycle where primary oocytes ovulate after 2 days of LH peak and then mature 2-3 days later, secondary oocytes last 2-3 days (Concannon et al, 1977). Lastly, diestrus is reached. Characterised by diminishing attraction of male dogs and resolving of vulvar oedema discharge, diestrus is the stage of refraction from breeding and our point of interest regarding PSPG as this is where it occurs as an exaggerated response (Blendinger, 2007).
It is also important to point out that the canine is unique in that, the state of pregnancy or non-pregnancy has no difference in the duration of reproductive cycle stages, development of the mammary gland and values of serum estradiol and progesterone concentrations (Romagnoli, 2009). In addition, Romagnoli (2009) also pointed out, however, that there will be considerable differences in hormonal values as seen in the data in figure 1.
Stage |
Duration |
Progesterone |
Estrogen |
Notes |
Anestrus |
1-6 months |
Basal level (<1ng/mL) |
Basal level (2-10 pg/mL) |
Ovarian inactivity with no overt vulvar discharge |
Proestrus |
3 days - 3 weeks (9 days average) |
Initially basal; 2-3 ng/mL at LH surge; 4-10 ng/mL at day of ovulation |
Rising to peak levels (50-100 pg/mL) |
Vulvar discharge present and vulva mildly enlarged and vulval oedema at maximal. Progesterone is seen in circulation. |
Estrus |
5-15 days |
Progesterone level rises in circulation |
Abrupt decrease at the day of LH peak to 10-20 pg/mL, then Basal level (2-10pg/mL) during the following few days |
Primary oocytes ovulate 2 days after the LH peak, oocyte maturation is seen 2-3 days later, lifespan of secondary oocytes is 2-3 days |
Diestrus |
2-3 months |
Peaks at 15-80 ng/ml then declines in late diestrual phase |
Basal (2-10 ng/mL) |
Resolved vulvar discharge and oedema. Prolactin levels increase in a reciprocal fashion to progesterone which results in enlarged mammary glands. |
Figure 1: Aspects of the estrous cycle in the bitch. Adapted from Concannon (1986); Blendinger (2007); Root Kustritz (2012).
Causes
Although not everything is known about what causes PSPG, It has been suggested that sensitivity of the endometrium and mammary glands to progesterone (P4), the increase in prolactin due to progesterone’s abrupt decrease and other hormones are the culprits behind pseudocyesis occurring in the late luteal phase of diestrous (Grunau et al, 1996).
Since PSPG is normally seen around 6-12 weeks after heat, at the expected whelping time, and the anti-prolactin agents were successful in the treatment of this syndrome, it is clear why these theories bear weight (Jochle et al, 1989).
What is known for sure, is that all the hormonal changes in the estrous cycle are normal and required in order to prepare for pregnancy, and because they are in circulation for a few weeks even if unfertilised these changes deceive the body into thinking it is pregnant. In addition, the non-pregnant dog has a corpus luteum lifespan that exceeds that in pregnant dogs (Gobello et al, 2001). Moreover, factors including nutrition, breed type, age and number of prior pregnancies also play a role (Johnston, 1980; Gobello et al, 2002).
a) Physiology of progesterone
|
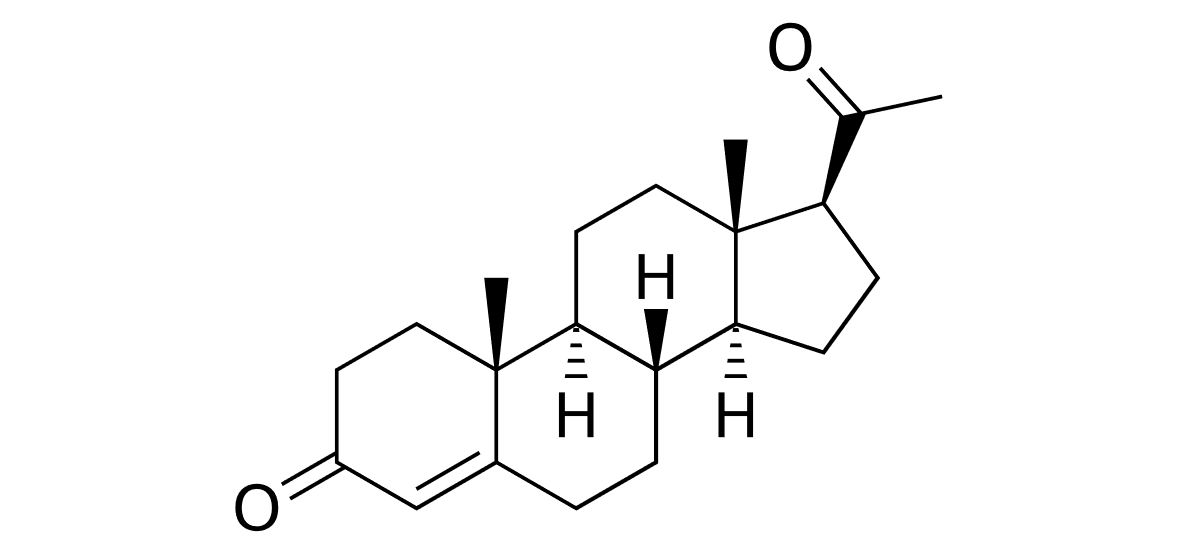
Figure 2 Structure of Progesterone |
Progesterone, whose structure can be seen in figure 2, is a steroid hormone that regulates ovulation and menstruation. It is produced in the corpus luteum of the ovaries which remains functional in the non-pregnant bitch for an extended period after ovulation (Gobello et al, 2001).
Due to the fact that LH induces its secretion, progesterone peaks at the late luteal phase and its concentration is equal in pregnant and non-pregnant bitches since they have no maternal recognition of pregnancy (Gobello et al, 2002; Concannon, 2011). Gobello et al (2001) highlighted the following effects of progesterone on the female sexual cycle:
- Promotes implantation and maintains a healthy pregnancy
- Thickens the vaginal epithelium and cervical mucus making it impenetrable to sperm cells
- Inhibits lactation during pregnancy
- Blocks central estrogen effect as it is the physiological antagonist of oestrogen
A study found that serum progesterone concentration was higher in pseudopregnant than pregnant labradors during weeks 1 to 6 of gestation or PSPG. It was also recorded that mean serum estradiol concentrations in dogs experiencing this syndrome was drastically higher than those of gestating individuals through week 3 and the lowest values were only observed at week 5 (Chakraborty, 1987).
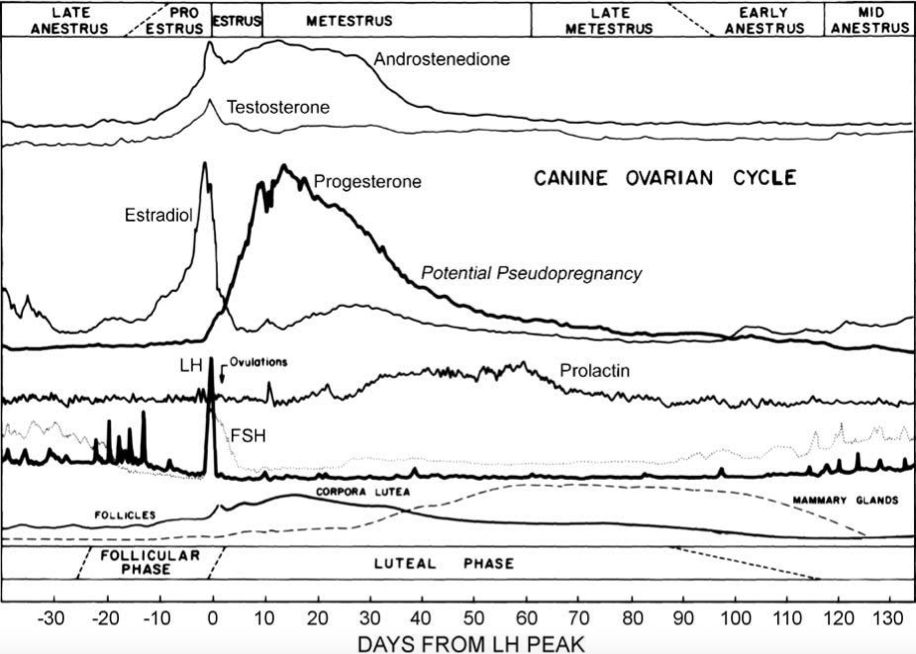
This shows that the reduction in progesterone coupled with its reversely proportional relationship to prolactin, which is illustrated in figure 4, is noteworthy when it comes to the causes of PSPG. Moreover, dogs who have been spayed during a time in their heat cycle where progesterone levels are high has been found to cause false pregnancy when progesterone rapidly drops after surgery (Johnston, 1986; Lee, 2006).
b) Physiology of prolactin
|
Figure 3 Structure of Prolactin |
Prolactin is a peptide hormone produced in the pituitary gland with its structure shown in figure 3. Secreted by lactotroph cells, it is an important hormone for the initiation and maintenance of lactation. Therefore, it is responsible for the enlargement of the mammary glands and activation of their ductal and glandular tissues (Gobello et al, 2001).
Increased prolactin serum concentrations, which occur when progesterone levels fall in the late luteal phase, inhibit the release of GnRH from the hypothalamus thereby decreasing the secretion of gonadotropins (Jochle, 1997). Its secretion is regulated by FSH and LH and cortical stimuli during lactation, while dopamine is said to be prolactin’s antagonist as it acts on D2 type dopamine receptors on the lactotroph cells (Egli et al, 2010).
However, Gobello et al (2001) and Tsutsui et al (2007) both reported a lack of relationship between plasma prolactin and clinical PSPG, which is why studies suggest that individual sensitivity to prolactin plays a role in PSPG. In addition, its association to progesterone decrease depicted in figure 4, as well as molecular variations of canine prolactin which exhibit different bioactivity from one another may help clarify the etiopathology of this syndrome (Gobello et al, 2002).
|
Figure 4 Schematic of regular changes in concentrations of reproductive hormones during the estrous cycle of the bitch (Concannon, 2011) |
c) Causes in goats
Pseudopregnancy in goats occurs due to a persistent corpus luteum. It can occur even in unmated animals out of the breeding season. Furthermore, when animals mate out of the breeding season the likelihood of the syndrome developing increases (Taverne, 2020).
The persistent corpus luteum secretes progesterone which causes hydrometra. Another hormone which results in hydrometra is prolactin. Its role in hydrometra is supported by several reasons. First, prolactin forms part of the luteotrophic complex in cyclic does (Buttle, 1983).
Secondly, udder development and lactogenesis occur during the last part of pseudopregnancy and around the cloudburst (discharge of uterine fluid) in non-lactating does. This, along with the distended abdomen, gives the animal a pregnant appearance.
Lastly, prolactin is involved in the fluid accumulation during pregnancy, chiefly at the interfaces of the endometrium and foetal membranes (De Bakker, 1986).
d) Causes in humans
There is no one specific answer to why women experience a PSPG but many different studies may shed light to a few reasons of why a woman might go through such an experience. Many women experiencing pseudocyesis nowadays may be found mostly in rural undeveloped countries. In these countries, there would probably be a lack of physicians or midwives. Therefore women who believe they are pregnant would not go to get examined until they would be ‘in labour’. This comes hand in hand with both a lack of education and poverty. When speaking about pregnancy nowadays it seems to be the norm that everyone would know pregnant women must get examined a few times during their gestation period. In more developed countries women visit obstetricians in the first trimester of pregnancy, due to this they would have an accurate idea about the pregnancy and the embryo (Pawlowski and Pawlowski, 2013).
It is important to differentiate between pseudocyesis and delusion of pregnancy. The delusion of pregnancy is linked with schizophrenic people or people suffering from some other sort of psychological disorder. This refers to the false belief of pregnancy without any symptoms experienced by psychotic women or even sometimes men (Brooks, 2016).
Pseudopregnancy may also be induced when a woman has an intense desire to get pregnant. This may be due to repeat miscarriages, infertility or even incoming menopause. Due to this worry, a woman's body may start producing signs of pregnancy. Therefore the woman's brain will misinterpret these signs as symptoms of pregnancy. This causes a trigger to release certain hormones of pregnancy like prolactin and estrogen and may lead to actual symptoms of pregnancy (Gabbe et al, 2007).
Data from a recent review indicates that pseudocyesis in women may have a deficit in brain dopamine activity. This fact supports the notion that pseudopregnant women may have a dysfunction of the central nervous system in their catecholaminergic pathways involved in the regulation of anterior pituitary hormone secretion. Dopamine and norepinephrine have been recently identified in mice as potent steroid-independent inhibitors of gonadotropin-releasing hormone (GnRH) neuron excitability and firing. Moreover, dopamine in women inhibits pulsatile LH and PRL. Thus, a deficit/dysfunction in the brain's catecholaminergic activity may result in increased pulsatile GnRH, LH and PRL and an elevated LH/FSH ratio (Jaffe et al, 1990; Ben-Jonathan and Hnasko, 2001; Liu and Herbison, 2008.2013).
Clinical signs
All non-pregnant bitches in mid and late diestrus can either show no clinical signs, i.e., covert PSPG, or have a wide range of observable clinical signs, i.e., overt PSPG. Firstly, this syndrome usually manifests itself in behavioural changes that include restlessness, decreased activity, anorexia, aggression and licking of the abdomen (Gobello et al, 2001).
In addition, Romagnoli (2009) highlighted maternal behaviour that includes carrying of inanimate objects and puppies that don’t belong to her and also a nesting habit (Romagnoli, 2009). Later, physical signs such as mammary enlargement accompanied by milk secretion, weight gain and contractions of the abdomen that copy those of parturition can be observed (Mialot et al, 1984; Feldman and Nelson, 1996).
Romagnoli (2009) also pointed out ancillary signs such as diarrhoea, polyuria (excessive urine production) and polyphagia (increased appetite and feeding). Furthermore, mastitis and mammary dermatitis are known for being uncommon clinical complications of PSPG. However, if these do not appear, clinical signs of this syndrome usually stop after 2-4 weeks (Johnston, 1986).
It was also found that susceptible bitches have a high recurrence rate in successive oestrus cycles (Feldman and Nelson, 1996). The risk of mammary tumours development related to the frequency of pseudopregnancies is illustrated in Figure 5 (Donnay et al, 1994).
Pseudopregnancy Frequency |
Dogs with history of PSPG with tumours |
Dogs with history of PSPG without tumours |
Odds ratio (IC 95%) |
<3, non-systematic |
108 |
158 |
1.5 (0.99-2.3) |
>3, systematic |
73 |
109 |
1.9 (1.15-3) |
Total |
181 |
267 |
1.6 (1.14-2.3) |
Figure 5: Odds ratio for the risk of mammary tumours development related to the frequency of pseudopregnancies (Donnay et al, 1994)
a) Clinical signs in goats
The primary clinical sign is hydrometra which is also called mucometra (Reddy et al, 2014). It is the accumulation of fluid in the uterus. This symptom, as discussed under the section ‘Causes’ occurs due to high progesterone levels and prolactin. If not diagnosed, the fluid can stay in the animal for several months and its volume rises up to litres.
This large volume of fluid causes the abdomen to distend and gives the appearance of pregnancy (Taverne, 2020). This can be accompanied by udder enlargement. Its risk increases with milk yield and the age of the dam. Hydrometra is rare in young goats (Taverne et al, 1995).
b) Clinical signs in humans
When a woman is passing through a PSPG she would experience many of the symptoms similar to that of an actually pregnant woman. These include:
- Swollen abdomen (lower belly)
- Nausea and vomiting
- Enlarged breasts and differences in the nipples (colour, size)
- Weight gain
- A disruption of the menstrual cycle
- Lactation
- Feeling imaginary foetal movements
- Increased appetite
- labour pains (rare)
During this syndrome an explanation to why the woman might experience something similar to a ‘baby bump’ is just a swollen belly due to the buildup of - gas, fat faeces and urine. If the doctors don't give the women a proper check-up they could also start believing that these women are pregnant as it is very hard to distinguish the symptoms from an actual pregnancy (Ibekwe, 2008; Yadav, 2012; Tarin et al, 2013).
Diagnosis
Proper and accurate diagnosis of PSPG is of utmost importance. This is because if an actually pregnant bitch is misdiagnosed with pseudopregnancy and is prescribed prolactin antagonist compounds these could result in an abortion or preterm whelping. Prior to diagnosing a bitch with this syndrome, Thangamani et al (2018) highlighted the following clinical steps that must be followed:
1. Make sure to check all clinical signs and ensuring they are in fact due to a pseudopregnancy and not due to a real pregnancy.
2. Abdominal palpation - if the bitch is actually pregnant the foetal part is palpable.
3. Ultrasound if the bitch is in an early diestrual stage.
4. Radiograph if the bitch is in a late diestrual stage.
5. Hormone estimation:
- The relaxin hormone can not be detected in a non-pregnant bitch.
- The thyrotrophic releasing hormone is responsible for the release of prolactin and thyrotrophin. If it is detected this could possibly be associated with hypothyroidism.
- Acute-phase protein is not present in a non-pregnant bitch.
a) Diagnosis in goats
PSPG can be diagnosed by transcutaneous ultrasonographic scanning of the pre pelvic area of the abdominal cavity. It is the most adequate diagnostic tool for managing reproduction (Gonzalez-Bulnes et al, 2010).
This is because it is a straightforward, non-invasive and fast method that allows the approximation of litter size and foetal weight (Santiago-Moreno et al, 2005). Hydrometra is usually diagnosed during routine pregnancy diagnosis of mated animals.
The fluid is seen as black spots of different sizes separated by thin double layers of tissue. If a great amount of fluid is present, the layers of the tissue show undulation when the abdominal wall is shaken. However, it is important to keep in mind that hydrometra can also occur in unmated anoestrous does (Taverne, 2020).
In the early stages, it is difficult to differentiate between normal pregnancy and PSPG i.e. hydrometra. This is because the foetus would anyway be too small to be seen at this point. Furthermore, the accumulated fluid could be mistaken for allantoic fluid. A conclusion can be drawn thirty days postmating anestrus as the level of glycoproteins in the peripheral blood increases in the case of an actual pregnancy (Taverne, 2020).
b) Diagnosis in humans
When diagnosing a woman with PSPG many different approaches may be taken. The doctor will first go about evaluating her symptoms as he would do to a normal pregnant woman. The doctor will then go on with the usual tests which would be performing a pelvic exam and abdominal ultrasound. Unlike a normal pregnancy, the doctor will not observe anything in the ultrasound except an empty abdomen and obviously no heartbeat will be present either (Gabbe et al, 2007).
When making a proper diagnosis of a pseudopregnancy it is important to give the woman proof that she is not pregnant. There are two types of pregnancy tests which can be administered, a urine test and a blood test. Both of these tests will detect the human chorionic gonadotropin hormone. This hormone is secreted by the trophoblast cells of the placenta. This process takes place after detachment of the embryo from the uterus lining. After this happens the hormone will build up very quickly all over the body and therefore can be tested during the pregnancy test. If a woman is pseudopregnant this hormone will not be detected and therefore the test’s outcome will be negative (Chard, 1992).
Treatment strategies
If the case is mild, no treatment is needed except for discouraging maternal behaviour. If the behaviour persists other small preventive methods can be used. These include using an Elizabethian collar to prevent the licking of mammary glands; avoiding milking, licking and use of compresses as these stimulate lactation; removing water for a period of five to seven days at night so as to promote fluid consumption which helps end lactation. However, the latter requires that beforehand, normal renal function is checked (Feldman and Nelson, 1987).
If the behavioural signs are significant, non-phenothiazine drugs may be used, whose effect is light tranquillisation. On the other hand, phenothiazine drugs should not be used in pseudopregnant bitches as they stimulate prolactin secretion (Voith, 1983).
It is important to note that treatment in mild cases is suggested in the case that it’s not the first time that the bitch is pseudopregnant. This is due to the possibility that pseudopregnancy is correlated with mammary tumours that develop afterwards, according to recent reports (Verstegen, 1999).
In the event that the case is moderate to severe, sex steroids, including oestrogens, progestins and androgens may be used. Nonetheless, nowadays, the preferred method for treatment is dopamine-agonists, namely bromocriptine and cabergoline (Allen, 1986).
a) Sex steroids
Sex steroids were the traditional method to treat pseudopregnancy, yet, it was realised that their side effects outweigh any benefits they may have. Even though sex steroids are necessary for mammary development, when in high amounts they exert negative effects. These effects may result in either suppressing the production of the prolactin by the pituitary gland or by decreasing the sensitivity of the body towards prolactin.
Oestrogens: Their effects might be signs of proestrus and estrus, uterine diseases and bone marrow depression which results in anaemia.
Androgens: These put an end to lactation, they cause clitoral hypertrophy, other forms of virilisation and epiphora.
Progestins: These are used to suppress the symptoms of overt pseudopregnancy. This mechanism is unknown but it most likely involves the above-mentioned effects on prolactin. Their side effects are cystic endometrial hyperplasia-pyometra complex, resistance to insulin, mammary gland nodules, mammary tumours and acromegaly (Feldman and Nelson, 1987).
b) Dopamine-agonists
These include bromocriptine and cabergoline and have the role of inhibiting prolactin secretion (Ben-Jonathan and Hnasko, 2001). Dopamine agonists are released by three neural groups of the hypothalamus namely periventricular dopaminergic, tuberohypophyseal and tuberoinfundibular neurons (Egli et al, 2010).
The suprachiasmatic nucleus is the mammalian biological clock as it controls all activities that are concerned with the circadian rhythm (Reppert and Weaver, 2002).
Thus, its neurons influence the dopamine neurons. The suprachiasmatic nucleus is the starting point for the vasoactive intestinal peptide (VIP) fibres. These innervate the arcuate nucleus as well as the periventricular nucleus which results in the excitation of the dopamine neurons (Egli et al, 2010).
Prolactin is secreted by ergot alkaloid drugs from the pituitary gland. It is under an extensive set of stimulatory and inhibitory factors as well as hormones that originate both peripherally and centrally. The secretion is inhibited by the tonic control of the hypothalamus mediated by dopamine which is the major prolactin inhibiting factor (PIF). Prolactin also regulates its own secretion by negative feedback. This mechanism involves the stimulation of dopamine (DA) neurons (Egli et al, 2010).
This results in dopamine secretion, due to the elevation in the concentration of prolactin, thus inhibiting its own prolactin secretion (Bertram, 2005). The hypothalamus can be relieved from its inhibition by serotonin. This means that dopamine release is suppressed while prolactin secretion is stimulated (Thorner et al, 1998).
Two other substances that stimulate prolactin secretion, as well as thyroid-stimulating hormone secretion, are hypothalamic tripeptide and thyrotropin release hormone (TRH). On the other hand, serotonin can be inhibited by metergoline, an ergot alkaloid (Janssens, 1986). Its side effects include anxiety, aggressiveness, hyperexcitation and whining (Peterson and Drucker, 1981). Dopamine agonists have a direct effect on D2-dopamine receptors of the lactotroph cells of the anterior pituitary gland.
Cabergoline
Cabergoline is better than bromocriptine as it has greater bioactivity, it is more specific to the D2-receptor and its actions last for a longer period of time. The blood-brain barrier is only slightly permeable to it, thus, it has less central emetic effects when compared to bromocriptine (Arbeiter et al, 1988).
Bromocriptine
In certain countries, it is labelled as a drug in human medicine but not in veterinary medicine. With that being said, it has been used as an extra-label and experimentally in veterinary medicine. Bromocriptine is less specific than cabergoline as it also acts on the GABA, serotonergic and adrenergic receptors. Stimulation of the hypothalamic vomiting centre results in emetic effects. The side effects are anorexia, vomiting and depression; however, their severity decreases as treatment progresses.
c) Treatment strategies in goats
Treatment is successful once the uterine fluid is discharged (so-called cloudburst) from the pseudopregnant goat (MAM Taverne et al, 1995). This discharging can be brought about by treatment with prostaglandin-F2⍺ (Pieterse and Taverne, 1986).
d) Treatment strategies in humans
Generally, when a woman experiences a pseudopregnancy it is not normal that she will show any physical signs, therefore medication is not usually prescribed. An exception could be if the woman is passing through a time of menstrual irregularity. For some women it may be very upsetting to find out that they are in fact not pregnant after strongly believing that they were, they might not even want to believe their doctor. Therefore it is very important to provide the patient with physical proof to help her get over the fact that she has no foetus. This may be done by showing her the ultrasound taken and in fact, is a very successful way of ending this pseudopregnancy (Ibekwe, 2008; Yadav, 2012; Tarin et al, 2013).
Preventive methods
The only preventative method which can be taken to prevent a pseudopregnancy in dogs is to conduct an ovariohysterectomy. Normally bitches which are not intended for breeding purposes should be spayed. The spaying should be done during the anestrus. Spaying during lactation could lead to a prolonged pseudopregnancy (Concannon et al, 2001).
If the bitch is spayed during the diestrual period or during a pseudopregnancy could result in a decline in plasma progesterone and an incline in plasma prolactin increase which would make the pseudopregnancy become overt and last even longer (Thangamani et al, 2018).
References
Allen, W. E. (1986): Pseudopregnancy in the bitch. The current view on aetiology and treatment. J Small Anim Pract 27: p. 419- 424.
Arbeiter, K.; Brass, W.; Ballabio, R. (1988): Treatment of pseudopregnancy in the bitch with cabergoline, an ergoline derivative. J Small Anim Pract 29: p. 781-788.
Ben-Jonathan, N.; Hnasko, R. (2001): Dopamine as a Prolactin (PRL) Inhibitor. Endocrine Reviews 22: (6) p. 724-763.
Bertram, R. (2005): A mathematical model for the mating-induced prolactin rhythm of female rats. AJP: Endocrinology and Metabolism, 290: (3) p. 573-582.
Blendinger, K. (2007): Physiology and pathology of the estrous cycle of the bitch. IVIS 56th Congresso Internazionale Multisala SCIVAC: p. 73-77.
Buttle, H. L. (1983): The luteotrophic complex in hysterectomized and pregnancy goats. J Physiol 482: p. 399-407.
Chakraborty, P. (1987): Reproductive hormone concentrations during estrus, pregnancy, and pseudopregnancy in the Labrador bitch. Theriogenology 27: (6) p. 827-840.
Chard, T. (1992): Pregnancy tests: a review. Hum Reprod.7: (5) p. 701–710.
Concannon, P. W.; England, G.; Verstegen, J. (Eds.) (2001): Recent Advances in Small Animal Reproduction, (www.ivis.org). Publisher: International Veterinary Information Service, Ithaca, New York, USA.
Concannon, P.W. (2011): Reproductive cycles of the domestic bitch. Anim Reprod Sci. 124: p. 200–10.
De Bakker, O. J. G. B. (1986): Human amniotic fluid prolactin and the fetal membranes. PhD thesis, Faculty of Medicine, University of Amsterdam.
Egli, M. (2005): Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. AJP: Endocrinology and Metabolism 290: (3) p. E566-E572.
Egli, M.; Leeners, B.; Kruger, T. (2010): Prolactin secretion patterns: basic mechanisms and clinical implications for reproduction. Reproduction, 140: (5) p. 643-654.
Feldman, E. C.; Nelson, R. W. (1987): Canine and Feline Endocrinology and Reproduction. Philadelphia: WB Saunders Co.
Feldman, E. C.; Nelson, R. W. (1996): Canine and Feline Endo-crinology, Reproduction, 2nd edn. WB Saunders, Philadelphia, USA.
Gabbe, S. G.; Niebyl, J. R.; Simpson, J. L.; eds. Obstetrics (2007): Normal and Problem Pregnancies, fifth ed., Philadelphia, Churchill Livingstone Elsevier.
Gobello, C.; Colombani, M.; Scaglia, H.; De La Sota, R. L.; Goya, R. (2001): Heterogeneity of circulating prolactin in the bitch. Reprod. Nutr. Dev,41: (6) p. 505-511.
Gobello, C.; De La Sota, R. L.; Luzbel, R.; Goya, R. (2002): A Review of Canine Pseudocyesis. Reproduction in domestic animals = Zucht Hygiene 36: p. 283-8.
Gobello, C.; De La Sota, R. L.; Castex, G.; Baschar, H.; Goya, R. G. (2001): Diestrous ovariectomy: a model to study the role of progesterone in the onset of canine pseudopregnancy.J Reprod Fertil.
Gobello, C.; De La Sota, R. L.; Goya, R. G. (2001): Study of the change of prolactin and progesterone during dopaminergic agonist treatments in pseudopregnant bitches. Anim Reprod Sci. 66: (2) p. 57–67.
Gonzalez-Bulnes, A.; Pallares, P.; Vazquez, M. (2010): Ultrasonographic imaging in small ruminant reproduction. Reprod. Domest. Anim. 45: p. 9–20.
Grunau, B.; Nolte, I.; Hoppen, H. O. (1996): Investigation on the treatment of pseudopregnancy in the bitch with the prolactin inhibitors metergoline and bromocriptine. Tierarztl Prax 24: p. 149-155.
Günzel‐Apel, A.; Urhausen, C.; Wolf, K.; Einspanier, A.; Oei, C.; Piechotta, M. (2012): Serum Progesterone in Pregnant Bitches Supplemented with Progestin Because of Expected or Suspected Luteal Insufficiency. Reprod Domest Anim, 47: p. 55-60.
Ibekwe, P. (2008): Psychosocial and cultural aspects of pseudocyesis. Indian Journal of Psychiatry, 50: (2) p. 112-116.
Janssens, L. A. (1986): Treatment of pseudopregnancy with bromocriptine, an ergot alkaloid. Vet Rec 119: p. 172-174.
Jochle, W.; Ballabio, R.; diSalle, E. (1987): Inhibition of lactation in the beagle bitch with the prolactin inhibitor cabergoline: Dose response and aspects of long term safety. Theriogenology 27: p. 799-810.
Jochle, W.; Arbeiter, K.; Post, K.; Ballabio, R.; D'ver, A. S. (1989): Effects on pseudopregnancy, pregnancy and interestrous interval of pharmacological suppression of prolactin secretion in female dogs and cats. Journal of Reproduction and Fertility, Supplement 39: p. 199-207.
Jöchle, W. (1997): Prolactin in canine and feline reproduction. Reprod Domest Anim. 32: p. 183–93.
Johnston, S. D. (1980): False pregnancy in the bitch. In: Morrow DA, ed. Current Veterinary Theriogenology. Philadelphia: WBSaunders CO.: p. 623-624.
Lee, W. M.; Kooistra, H. S.; Mol, J. A.; Dieleman, S. J.; Schaefers-Okkens, A. C. (2006): Ovariectomy during the luteal phase influences secretion of prolactin, growth hormone, and insulin-like growth factor-I in the bitch. Theriogenology 66: (4) p. 84–90.
Paul, M.; Majumder, S.; Bhadra, A. (2014): Grandmotherly care: a case study in Indian free-ranging dogs. Journal of Ethology, 32: (2) p. 75-82.
Peterson, M. E.; Drucker, W. D. (1981): Advances in the diagnosis and management of canine cushing syndrome. In: Proceedings of the 31st Gaines Veterinary Symposium 17.
Pieterse, M. C.; Taverne, M. A. M. (1986): Hydrometra in goats:diagnosis with real-time ultrasound and treatment with prostaglandins and oxytocin. Theriogenology 216: p. 813-821.
Reddy, R.; Arunakumari, G.; Anil, K. R.; Muralimohan, K.; Sunil, A. (2014): Efficacy of cloprostenol therapy in hydrometra goats. Indian J. Anim. Reprod. 35: p. 39–41.
Reppert, S.; Weaver, D. (2002): Coordination of circadian timing in mammals. Nature, 418: (6901) p. 935-941.
Romagnoli, S. (2009): An Update on Pseudopregnancy. World small animal veterinary association world congress proceedings. Theriogenology, 2nd edn. WB Saunders, Philadelphia, USA: p. 490-491.
Root, A. L.; Parkin, T. D.; Hutchison, P. (2018): Canine pseudopregnancy: an evaluation of prevalence and current treatment protocols in the UK. BMC Vet Res 14: p. 170.
Root Kustriitz, M. V. (2012): Managing the reproductive cycle in the bitch. Vet Clin Small Anim. 42: p. 423–437.
Santiago-Moreno, J.; Gonzalez-Bulnes, A.; Gomes-Brunet, A.; Toledano-Diaz, A.; Lopez-Sebastian, A. (2005): Prediction of gestational age by transrectal ultrasonographic measurements in the Mouflon (Ovis gmelini musimon) J. Zoo Wildl Med. 36: p. 457–462.
Singh, L.; Bhimte, A.; Pipelu, W.; Kumar, M.; Kumar, G.; Kumar, P. (2018): Canine pseudopregnancy and its treatment strategies. Journal of entomology and zoology studies 6.
Souza, J. M.; Maia, A. L.; Brandao, F. Z.; Vilela, C. G.; Oba, E.; Bruschi, J. H.; Fonseca, J.F. (2013): Hormonal treatment of dairy goats affected by hydrometra associated or not with ovarian follicular cyst. Small Rumin. Res. 111: p. 104–109.
Steinetz, B. G.; Goldsmith, L. T.; Harvey, H. J.; Lust, G. (1989): Serum relaxin and progesterone concentrations in pregnant, pseudopregnant, and ovariectomized, progestin-treated pregnant bitches: detection of relaxin as a marker of pregnancy. Am J Vet Res. 50: (1) p. 68-71.
Tarín, J. J.; Hermenegildo, C.; García-Pérez, M. A. (2013): Endocrinology and physiology of pseudocyesis. Reprod Biol Endocrinol 11: p. 39.
Taverne, M. A. M.; Hesselink, J. W.; Bevers, M. M.; van Oord, H. A.; Kornalijnslijper, J. E. (1995): Aetiology and Endocrinology of Pseudopregnancy in the Goat 30: p. 228-230.
Taverne, M. A. M. (2020): Pseudopregnancy in Goats, MSDVetManual.
Thangamani, A.; Srinivas, M.; Chandra Prasad, B. (2018): Canine Pseudopregnancy: A Review. Research & Reviews: Journal of Veterinary Science and Technology. 7: (1) p. 7–11.
Thorner, M. O.; Vance, M. L.; Laws, R. E. (1998): The anterior pituitary. In: Wilson, J. D.; Foster, D. W.; Kronenberg, H. M.; et al. eds.: Williams Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders Co.: p. 249-340.
Upadhyay, S. (2008): Pseudocyesis. JNMA J Nepal Med Assoc. 47: (171) p. 147–150.
Verstegen, J. (1999): IntÈrÍt et applications des antiprolactiques en la cancÈrologie chez la chienne. In: Proceedings of the 24th World Small Animal Veterinary Congress Paris, France.
Voith, V. L. (1980): Functional significance of pseudocyesis. Mod Vet Pract: p. 61-75.
Voith, V. L. (1983): Behavioral disorders. In: Ettinger SJ, ed. Textbook of Veterinary Internal Medicine. 2nd ed. Philadelphia: WB Saunders Co.: p. 513-522.
Weber, A. F. (1944): "Pseudopregnancy In Dogs," Iowa State University Veterinarian: Vol. 7 : Iss. 1 , Article 6.
Yadav, T. (2012): Pseudocyesis versus delusion of pregnancy: differential diagnoses to be kept in mind. Indian Journal of Psychological Medicine, 34: (1) p. 82-84.
Figures
Figure 1: Aspects of the estrous cycle in the bitch. Adapted from Blendinger (2007); Concannon (1986); Root Kustritz (2012).
Figure 2: Structure of Progesterone (en.wikipedia.org)
Figure 3: Structure of Prolactin (https://www.pinterest.com/pin/19984792076174747/)
Figure 4: Schematic of regular changes in concentrations of reproductive hormones during the estrous cycle of the bitch (Concannon, 2011)
Figure 5: Odds ratio for the risk of mammary tumours development related to the frequency of pseudopregnancies (Donnay et al 1994)