|
Size: 22057
Comment:
|
Size: 22053
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 102: | Line 102: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0;"style="padding:0.5em;"> {{attachment:Structure of Prolactin.png||width="600"}} || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0;"style="padding:0.5em;"> {{attachment:Prolactin.png||width="600"}} || |
| Line 114: | Line 114: |
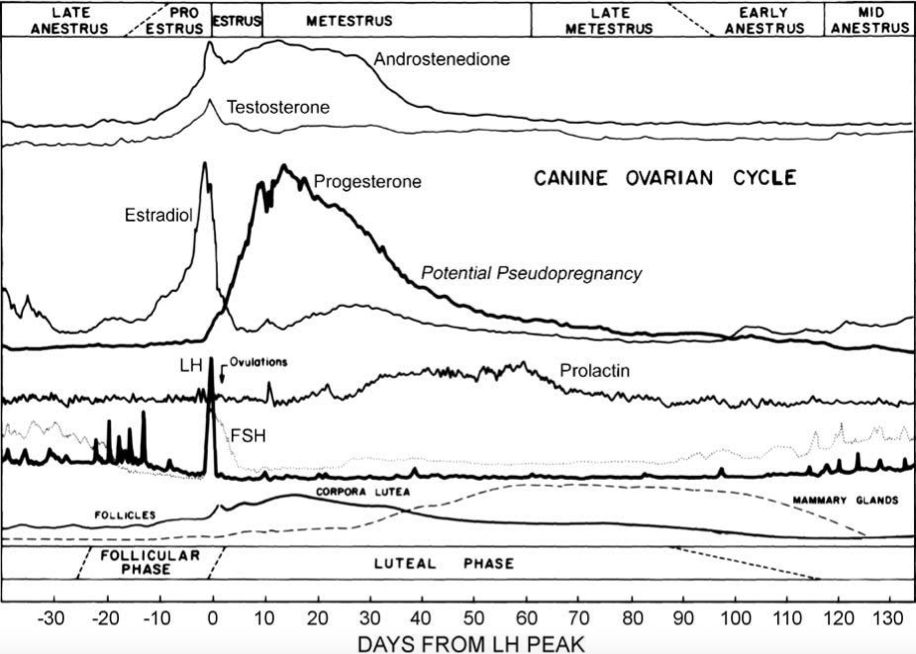
| '''' ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0;"style="padding:0.5em;"> {{attachment:Schematic of hormone levels in the estrous cycle of the bitch.png||width="600"}} ||||'''Figure 4''' Schematic of regular changes in concentrations of reproductive hormones during the estrous cycle of the bitch (Concannon, 2011) || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0;"style="padding:0.5em;"> {{attachment:Schematic of hormone levels in the estrous cycle of the bitch.png||width="600"}} || ||'''Figure 4'''Schematic of regular changes in concentrations of reproductive hormones during the estrous cycle of the bitch (Concannon, 2011) '''' || |
Itt írjon a(z) Pseudopregnancy-ról/ről
Canine Pseudopregnancy
Contents
Introduction
Canine Pseudopregnancy (PSPG), also known as pseudocyesis, nervous lactation, copycat pregnancy or phantom pregnancy, is a normal self-limiting physiological syndrome that occurs in female dogs in their late diestrual phase. Signs of pregnancy will be shown in the postpartum period, however, in reality, the bitch is not pregnant (Feldman and Nelson 1996; Gobello et al, 2001b). Behavioural and physical changes occur. These may be covert i.e. without any clinical signs, or overt i.e. with clinical signs and are seen between 6-8 weeks after estrus (Gobello et al. 2002).
Although its exact prevalence is not known, it has been estimated that its incidence rate is as high as 50-75% in certain breeds, using a broad definition of the condition (Johnston, 1980). Voith (1980) and Jochle (1987) shared the opinion through their work that PSPG evolved through families because of the need for non-mated wolves to lactate and feed other female wolves’ litters. This is called alloparental care in mammal species that live in groups, where submissive individuals care for the dominants’ offspring without reproducing themselves (Paul et al., 2014).
The purpose of this essay is to present the most relevant aspects of the physiology, clinical signs, diagnosis, treatment and prevention of clinical pseudopregnancy while also highlighting information regarding this syndrome in humans and goats.
a) Pseudopregnancy in Goats
Pseudopregnancy is a pathological condition that causes temporary infertility in goats (Souza J.M, Maia A.L, Brandao F.Z, Vilela C.G, Oba E, Bruschi J.H, Fonseca J.F., 2013). It is one of the major causes of anestrus, the absence of the expression of estrus, in dairy goats, thus leading to decreased reproductive activity (M. Taverne, February 2020).
b) Pseudopregnancy in Humans
The Canine Estrous Cycle
A good understanding of the canine estrous cycle and endocrinology is needed to fully grasp the concept of PSPG and the hormonal changes that cause it. The bitch is said to be monoestrous and has the onset of the first estrus between 6-10 months of age and goes through estrus every 6 months thereafter (Concannon, 2011; Gobello et al., 2001b).
The anestrus phase is marked by ovarian inactivity, endometrial repair and uterine involution and lasts about 6 months. Its termination is marked by the secretion of pituitary gonadotropins, follicle-stimulating hormone (FSH), luteinizing hormone (LH) induced by gonadotropin-releasing hormone (GnRH). The release of LH results in proestrus folliculogenesis (Blendinger, 2007).
This phase is then followed by proestrus, a phase averaging about 9 days where the vulva becomes enlarged and serosanguineous discharge can be seen. A prominent hormonal change of this phase is the LH peak which causes estrogen to decrease and subsequently increases progesterone steadily (Concannon et al., 1977). These changes result in the luteal phase of the ovarian cycle and therefore estrus is reached.
Estrus lasts about 9 days on average and is the part of the cycle where primary oocytes ovulate after 2 days of LH peak and then matures 2-3 days later, secondary oocytes lasts 2-3 days (Concannon et al. 1977). Lastly, diestrus is reached. Characterised by diminishing attraction of male dogs and resolving of vulvar oedema discharge, dioestrus is the stage of refraction from breeding and our point of interest regarding PSPG as this is where it occurs as an exaggerated response (Blendinger, 2007).
It is also important to point out that the canine is unique in that, the state of pregnancy or non-pregnancy has no difference in the duration of reproductive cycle stages, development of the mammary gland and values of serum estradiol and progesterone concentrations (Romagnoli, 2009). In addition, Stefano Romagnoli (2009) also pointed out that there will be considerable differences in hormonal values as seen in the data in Figure 1.
Stage |
Duration |
Progesterone |
Estrogen |
Notes |
Anestrus |
1-6 months |
Basal level (<1ng/mL) |
Basal level (2-10 pg/mL) |
Ovarian inactivity with no overt vulvar discharge |
Proestrus |
3 days - 3 weeks (9 days average) |
Initially basal; 2-3 ng/mL at LH surge; 4-10 ng/mL at day of ovulation |
Rising to peak levels (50-100 pg/mL) |
Vulvar discharge present and vulva mildly enlarged and vulval oedema at maximal. Progesterone is seen in circulation. |
Estrus |
5-15 days |
Progesterone level rises in circulation |
Abrupt decrease at the day of LH peak to 10-20 pg/mL, then Basal level (2-10pg/mL) during the following few days |
Primary oocytes ovulate 2 days after the LH peak, oocyte maturation is seen 2-3 days later, lifespan of secondary oocytes is 2-3 days |
Diestrus |
2-3 months |
Peaks at 15-80 ng/ml then declines in late diestrual phase |
Basal (2-10 ng/mL) |
Resolved vulvar discharge and oedema. Prolactin levels increase in a reciprocal fashion to progesterone which results in enlarged mammary glands. |
Figure 1: Aspects of the estrous cycle in the bitch.Adapted from Blendinger (2007); Concannon (1986); Root Kustritz (2012).
Causes
Although not everything is known about what causes PSPG, It has been suggested that sensitivity of the endometrium and mammary glands to progesterone (P4), the increase in prolactin due to progesterone’s abrupt decrease and other hormones are the culprits behind pseudocyesis occurring in the late luteal phase of diestrous (Grunau et al. 1996).
Since PSPG is normally seen around 6-12 weeks after heat, at the expected whelping time, and the anti-prolactin agents were successful in the treatment of this syndrome, it is clear why these theories bear weight (Jochle et al. 1989).
What is known for sure, is that all the hormonal changes in the estrous cycle are normal and required in order to prepare for pregnancy, and because they are in circulation for a few weeks even if unfertilised these changes deceive the body into thinking it is pregnant. In addition, the non-pregnant dog has a corpus luteum lifespan that exceeds that in pregnant dogs (Gobello et al., 2001b). Moreover, factors including nutrition, breed type, age and number of prior pregnancies also play a role (Johnston, 1980; Gobello et al, 2002).
a) Physiology of Progesterone
|
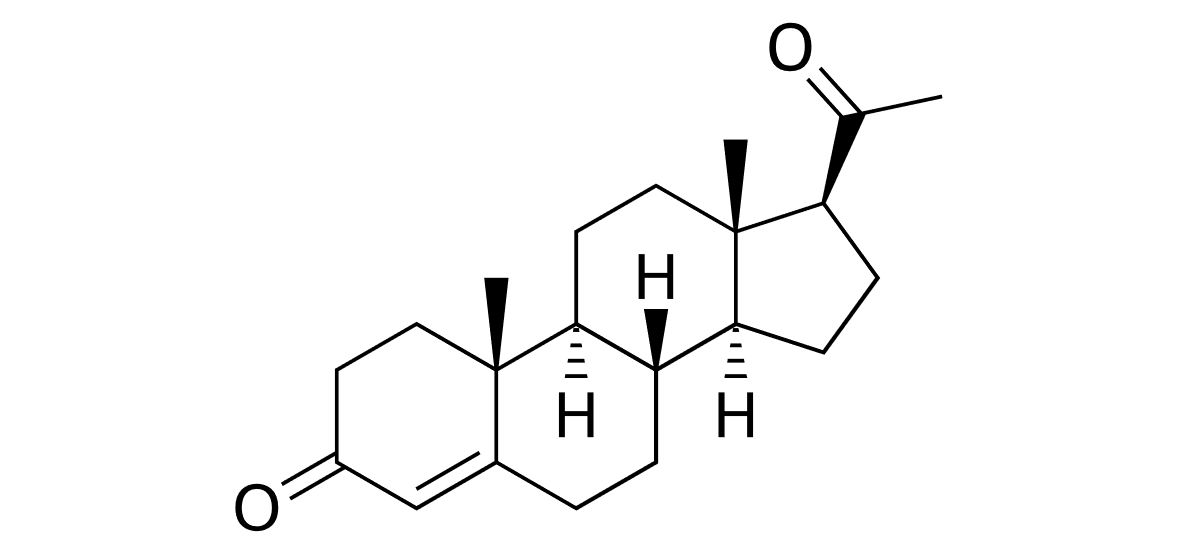
Figure 2 Structure of Progesterone |
Progesterone, whose structure can be seen in figure 2, is a steroid hormone that regulates ovulation and menstruation. It is produced in the corpus luteum of the ovaries which remains functional in the non-pregnant bitch for an extended period after ovulation (Gobello et al. 2001).
Due to the fact that its secretion is induced by LH, progesterone peaks at the late luteal phase and its concentration is equal in pregnant and non-pregnant bitches since they have no maternal recognition of pregnancy (Gobello et al. 2002; Concannon, 2011).
The effects of progesterone on the female sexual cycle are the following:
1. Promotes implantation and maintains a healthy pregnancy
2. Thickens the vaginal epithelium and cervical mucus making it impenetrable to sperm cells
3. Inhibits lactation during pregnancy
4. Blocks central Estrogen effect as it is the physiological antagonist of oestrogen (Gobello et al., 2001c)
A study found that serum progesterone concentration was higher in pseudopregnant than pregnant labradors during weeks 1 to 6 of gestation or pseudopregnancy. It was also recorded that mean serum estradiol concentrations in dogs experiencing pseudopregnancy was drastically higher than those of gestating individuals through week 3 and the lowest values were only observed at week 5 (Chakraborty, 1987).
This shows that the reduction in progesterone coupled with its reversely proportional relationship to prolactin, which is illustrated in Figure 4, is noteworthy when it comes to the causes of PSPG. Moreover, dogs who have been spayed during a time in their heat cycle where progesterone levels are high has been found to cause false pregnancy when progesterone rapidly drops after surgery (Johnston, 1986; Lee WM, 2006).
b) Physiology of Prolactin
|
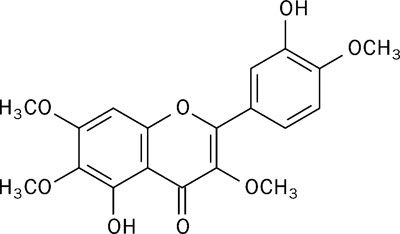
Figure 3 Structure of Prolactin |
Prolactin is a peptide hormone produced in the pituitary gland with its structure shown in figure 3. Secreted by lactotroph cells, it is an important hormone for the initiation and maintenance of lactation. Therefore, it is responsible for the enlargement of the mammary glands and activation of their ductal and glandular tissues (Gobello et al., 2001).
Increased prolactin serum concentrations, which occur when progesterone levels fall in the late luteal phase, inhibit the release of GnRH from the hypothalamus thereby decreasing the secretion of gonadotropins (Jochle, 1997). Its secretion is regulated by FSH and LH and cortical stimuli during lactation, while dopamine is said to be Prolactin’s antagonist as it acts on D2 type dopamine receptors on the lactotroph cells (Egli et al., 2010).
However, Gobello et al (2001) and Tsutsui et al (2007) both reported a lack of relationship between plasma prolactin and clinical PSPG, which is why studies suggest that individual sensitivity to prolactin, its association to progesterone decrease depicted in figure 4, as well as molecular variations of canine prolactin which exhibit different bioactivity from one another may help clarify the etiopathology of pseudopregnancy (Gobello et al, 2002).
|
Figure 4Schematic of regular changes in concentrations of reproductive hormones during the estrous cycle of the bitch (Concannon, 2011) ' |
c) Causes of Pseudopregnancy in Goats
Pseudopregnancy in goats occurs due to a persistent corpus luteum. It can occur even in unmated animals out of the breeding season. Furthermore, when animals are mated out of the breeding season the likelihood of pseudopregnancy to develop increases (M. Taverne, February 2020).
The persistent corpus luteum secretes progesterone which causes hydrometra. Another hormone which results in hydrometra is prolactin. Its role in hydrometra is supported by several reasons. First, prolactin forms part of the luteotrophic complex in cyclic does (Buttle, 1983).
Secondly, udder development and lactogenesis occur during the last part of pseudopregnancy and around the cloudburst (discharge of uterine fluid) in non-lactating does; this, along with the distended abdomen, gives the animal a pregnant appearance.
Lastly, prolactin is involved in the fluid accumulation during pregnancy, chiefly at the interfaces of the endometrium and foetal membranes (De Bakker, 1986).
d) Causes of Pseudopregnancy in Humans
Clinical Signs
All non-pregnant bitches in mid and late diestrus can either show no clinical signs i.e. covert pseudopregnancy, or have a wide range of observable clinical signs i.e. overt pseudopregnancy. Firstly, this syndrome usually manifests itself in behavioural changes, that include restlessness, decreased activity, anorexia, aggression and licking of the abdomen (Gobello et al. 2001).
In addition, Romagnoli (2009) highlighted maternal behaviour that include carrying of inanimate objects and puppies that don’t belong to her and also a nesting habit (Romagnoli, 2009). Later, physical signs such as mammary enlargement accompanied by milk secretion, weight gain and contractions of the abdomen that copy those of parturition can be observed (Mialot et al. 1984; Feldman and Nelson, 1996).
Romagnoli (2009) also pointed out ancillary signs such as diarrhoea, polyuria (excessive urine production) and polyphagia (increased appetite and feeding). Furthermore, mastitis and mammary dermatitis are known for being uncommon clinical complications of pseudopregnancy. However, if these do not appear, clinical signs of pseudopregnancy usually stop after 2-4 weeks (Johnston, 1986).
It was also found that susceptible bitches have a high recurrence rate in successive oestrus cycles (Feldman and Nelson, 1996). The risk of mammary tumours development related to the frequency of pseudopregnancies is illustrated in Figure 5 (Donnay et al., 1994).
Pseudopregnancy Frequency |
Dogs with history of PSPG with tumours |
Dogs with history of PSPG without tumours |
Odds ratio (IC 95%) |
<3, non-systematic |
108 |
158 |
1.5 (0.99-2.3) |
>3, systematic |
73 |
109 |
1.9 (1.15-3) |
Total |
181 |
267 |
1.6 (1.14-2.3) |
Figure 5: Odds ratio for the risk of mammary tumours development related to the frequency of pseudopregnancies (Donnay et al 1994)
a) Clinical Signs of Pseudopregnancy in Goats
The primary clinical sign is hydrometra which is also called mucometra (Reddy R et al., 2014). It is the accumulation of fluid in the uterus. This symptom, as discussed under the section ‘Causes’ occurs due to high progesterone levels and prolactin. If not diagnosed, the fluid can stay in the animal for several months and its volume rises up to litres. This large volume of fluid causes the abdomen to distend and gives the appearance of pregnancy. This can be accompanied by udder enlargement. Hydrometra is rare in young goats. Its risk increases with milk yield and the age of dam (M. Taverne, February 2020).
b) Clinical Signs of Pseudopregnancy in Humans
Diagnosis
a) Diagnosis in Goats
Pseudopregnancy can be diagnosed by transcutaneous ultrasonographic scanning of the pre pelvic area of the abdominal cavity. It is the most adequate diagnostic tool for managing reproduction (Gonzalez-Bulnes A, Pallares P, Vazquez M., 2010).
This is because it is a straightforward, non-invasive and fast method that allows the approximation of litter size and foetal weight (Santiago-Moreno J, Gonzalez-Bulnes A, Gomes-Brunet A, Toledano-Diaz A, Lopez-Sebastian A, 2005). Hydrometra is usually diagnosed during routine pregnancy diagnosis of mated animals.
The fluid is seen as black spots of different sizes separated by thin double layers of tissue. If a great amount of fluid is present, the layers of the tissue show undulation when the abdominal wall is shaken. However, it is important to keep in mind that hydrometra can also occur in unmated anoestrous does (M. Taverne, February 2020).
In the early stages, it is difficult to differentiate between normal pregnancy and pseudopregnancy i.e. hydrometra. This is because the foetus would anyway be too small to be seen at this point. Furthermore, the accumulated fluid could be mistaken for allantoic fluid. A conclusion can be drawn thirty days postmating anestrus as the level of glycoproteins in the peripheral blood increases in the case of an actual pregnancy (M. Taverne, February 2020).
b) Diagnosis in Humans
Treatment Strategies
If the case is mild, no treatment is needed except for discouraging the maternal behaviour. If the behaviour persists other small preventive methods can be used. These include using an Elizabethian collar to prevent the licking of mammary glands; avoiding milking, licking and use of compresses as these stimulate lactation; removing water for a period of five to seven days at night so as to promote fluid consumption which helps end lactation. However, the latter requires that beforehand, normal renal function is checked (Feldman EC and Nelson RW, 1987).
If the behavioural signs are significant, non-phenothiazine drugs may be used, whose effect is light tranquillisation. On the other hand, phenothiazine drugs should not be used in pseudopregnant bitches as they stimulate prolactin secretion (Voith VL,1983).
It is important to note that treatment in mild cases is suggested in the case that it’s not the first time that the bitch is pseudopregnant. This is due to the possibility that pseudopregnancy is correlated with mammary tumours that develop afterwards, according to recent reports (Verstegen J, 1999).
In the event that the case is moderate to severe, sex steroids, including oestrogens, progestins and androgens may be used. Nonetheless, nowadays, the preferred method for treatment is dopamine-agonists, namely bromocriptine and cabergoline (Allen WE, 1986).
Sex Steroids
Sex steroids were the traditional method to treat pseudopregnancy, yet, it was realised that their side effects outweigh any benefits they may have. Even though sex steroids are necessary for mammary development, when in high amounts they exert negative effects. These effects may result in either suppressing the production of the prolactin by the pituitary gland or by decreasing the sensitivity of the body towards prolactin.
Oestrogens: Their effects might be signs of proestrus and estrus, uterine diseases and bone marrow depression which results in anaemia.
Androgens: These put an end to lactation, they cause clitoral hypertrophy, other forms of virilisation and epiphora.
Progestins: These are used to suppress the symptoms of overt pseudopregnancy. This mechanism is unknown but it most likely involves the above-mentioned effects on prolactin. Their side effects are cystic endometrial hyperplasia-pyometra complex, resistance to insulin, mammary gland nodules, mammary tumours and acromegaly. (Feldman EC and Nelson RW, 1987)
Dopamine-Agonists
These include bromocriptine and cabergoline and have the role of inhibiting prolactin secretion (Ben-Jonathan and Hnasko, 2001). Dopamine agonists are released by three neural groups of the hypothalamus namely periventricular dopaminergic, tuberohypophyseal and tuberoinfundibular neurons (Egli et al., 2010).
The suprachiasmatic nucleus is the mammalian biological clock as it controls all activities that are concerned with the circadian rhythm (Reppert and Weaver, 2002).
Thus, its neurons influence the dopamine neurons. The suprachiasmatic nucleus is the starting point for the vasoactive intestinal peptide (VIP) fibres. These innervate the arcuate nucleus as well as the periventricular nucleus which results in the excitation of the dopamine neurons (Egli et al., 2010).
Prolactin is secreted by ergot alkaloid drugs from the pituitary gland. It is under an extensive set of stimulatory and inhibitory factors as well as hormones that originate both peripherally and centrally. The secretion is inhibited by the tonic control of the hypothalamus mediated by dopamine which is the major prolactin inhibiting factor (PIF). Prolactin also regulates its own secretion by negative feedback. This mechanism involves the stimulation of dopamine (DA) neurons (Egli et al., 2010).
This results in dopamine secretion, due to the elevation in the concentration of prolactin, thus inhibiting its own prolactin secretion (Bertram, 2005). The hypothalamus can be relieved from its inhibition by serotonin. This means that dopamine release is suppressed while prolactin secretion is stimulated (Thorner Mo, Vance ML, Laws RE, et al, 1998).
Two other substances that stimulate prolactin secretion, as well as thyroid-stimulating hormone secretion, are hypothalamic tripeptide and thyrotropin release hormone (TRH). On the other hand, serotonin can be inhibited by metergoline, an ergot alkaloid (Janssens LA, 1986). Its side effects include anxiety, aggressiveness, hyperexcitation and whining (Peterson ME and Drucker WD, 1981). Dopamine agonists have a direct effect on D2-dopamine receptors of the lactotroph cells of the anterior pituitary gland.
Cabergoline Cabergoline is better than bromocriptine as it has greater bioactivity, it is more specific to the D2-receptor and its actions last for a longer period of time. The blood-brain barrier is only slightly permeable to it, thus, it has less central emetic effects when compared to bromocriptine (Arbeiter K, Brass W, Ballabio R, et al., 1988)
Bromocriptine In certain countries, it is labelled as a drug in human medicine but not in veterinary medicine. With that being said, it has been used as an extra-label and experimentally in veterinary medicine. Bromocriptine is less specific than cabergoline as it also acts on the GABA, serotonergic and adrenergic receptors. Bromocriptine has a short half-life: approximately four to six hours. It should be administered at least twice a day for the best results. Stimulation of the hypothalamic vomiting centre results in emetic effects. The side effects are anorexia, vomiting and depression, however, their severity decreases as treatment progresses. Prevention or reduction of emesis is possible, however, it should be made sure that central dopamine blockers of synaptic transmission whose action would oppose that of bromocriptine are not used.
a) Treatment Strategies in Goats
Treatment is successful once the uterine fluid is discharged (so-called cloudburst) from the pseudopregnant goat. This discharging can be brought about by treatment with prostaglandin-F2⍺ (Pieterse and Taverne, 1986).
b) Treatment Strategies in Humans
Preventive Methods
References