Selenium
Contents
Introduction
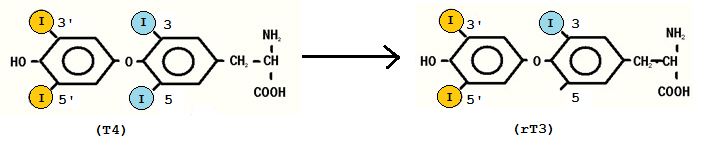
Selenium is one of many important elements needed for most animals to function normally. It is a basic element and has atomic number 34 in the periodic table (Murray et al, 2006), but is rarely found in elemental state in nature. The reason for this is that selenium often replaces sulphur in sulphur-containing substances. Selenium can be seen as an essential trace mineral found in soil and water. Plants absorb available selenium from the soil and incorporate it into amino acids by replacing the sulphur in cysteine and methionine (Figure 1). However, the normal function of the amino acids will stay the same independently on whether it contains sulphur or selenium (Christensen, 2006). In the body, selenium is known to have several important roles, like thioredoxin reductase, glutathione peroxidase and deiodinase that converts thyroxine (T4) to triiodothyronine (T3) (Murray et al, 2006). The approach in this essay will be how the selenium affects thyroid function and what the consequences are of selenium deficiency on the thyroid gland.
Selenoprotein
In animals approximately 25 different selenoproteins are known to have a biological function. By selenoprotein only proteins containing selenocysteine (Sec) are included (Saito et al, 2004). The selenoprotein is known to consist of 85 – 88 amino acids and is classified as a secreted glycoprotein. It is divided into two different domains according to the Sec content; the N-terminal domain contains 1 Sec, and the C-terminal domain contains 9 Sec. The selenoprotein has many isomers, which may differ in function depending on the type of isomer (Whanger et al, 2009). One of these selenoproteins is deiodinase, which is very important when it comes to the function and the synthesis of thyroid hormones (Kelly, 2000). In human thyrocytes, another selenoprotein, GPX3 (gluthatione peroxidase) is the most expressed one, and contributes to the high selenium content of the thyroid gland. Its main role in the body is to protect the cell membrane from free radicals, and is therefore considered as an important antioxidant. However, it plays a direct part in the thyroid hormone synthesis, by decreasing the amount of secreted H2O2 available for iodination reactions in the absence of TSH. Also reversely, if high level of TSH is present, the GPX3 will decrease, and more H2O2 will be available for TPO (thyroidal peroxidase) (Drutel et al, 2012). TPO is a lysosomal peroxidase enzyme system, which converts iodine to atomic iodine in the thyroid hormone synthesis (SzIE,Physiology Department et al, 2012).
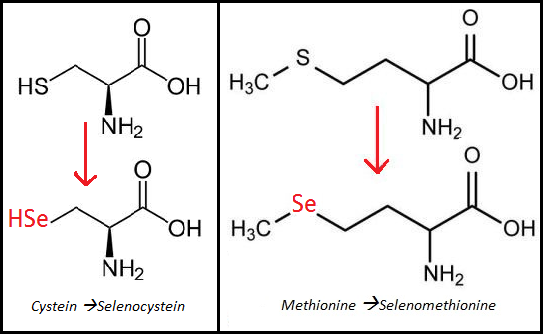
Figure 1: Selenium containing amino acids (Langaanes, 2012)
Thyroid
Thyroid gland produces hormones which act on every cell in the body. These hormones can be divided into three; T4, T3 and reverse triiodothyronine (rT3), where T4 and T3 are the most prominent ones. 90% of the thyroid secretion is T4, which is considered as the prohormone of T3 and rT3. It is distributed to different organs, and then synthesized to T3 by so called deiodinase enzymes. T3 is what we know as the active thyroid hormone, while rT3 is the inactive one (SzIE, Physiology Department et al, 2012).
Deiodination of thyroid hormones
Three deiodination families are known and are termed isoforms type I, II and III. The three families work in different tissues;
Type I: in liver, kidney, skeletal muscle and thyroid (Kelly, 2000), and is the source of about 80% of plasma T3 (Arthur et al, 1991)
- Type II: in brain, pituitary and brown adipose tissue
- Type III: in the brain, uterus, placenta and fetus.
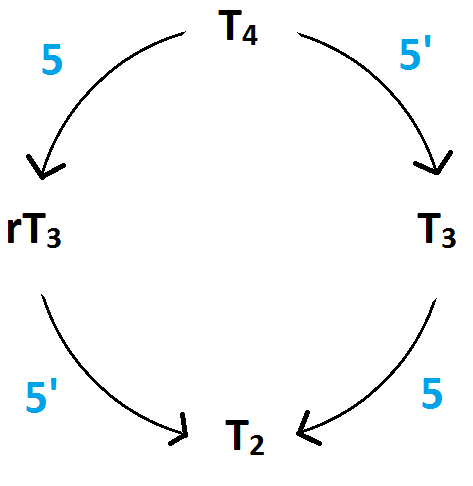
Figure 2: Synthesis of hormones (SzIE, Physiology Department et al, 2012), Figure 3: Tyrosyl rings of T4 and rT3 (SzIE, Physiology Department et al, 2012)
Type I is the most relevant for the thyroid function, where it can carry out both 5’ and 5- deiodination of T4 to produce either T3 or rT3. Type I 5’ requires selenium for the synthesis, while type I 5 is selenium independent. The majority of the activation of the T4 to the metabolically active T3 occurs through non-thyroidal deiodination (type I). T4 is secreted from the thyroid to the liver, where type I deiodinase activity may either result in T3 formation, iodine removal from the inner tyrosyl ring forming rT3 (Figure 3), or it can degrade it to T2 isomers (Figure 2). The T2 consists of two isomers; 3,5 T2 and 3,3 T2. Of the two, 3,5 T2 is the most active one and can only be formed from further deiodination of either T3 or rT3. Tests show that inhibition of the hepatic type I 5’ deiodinase enzyme, either due to selenium deficiency or some other influence, will lead to more degradation of rT3 to 3,3 T2 instead of the generation of T4 to T3. Stress, poor nutrition, illness and selenium deficiency are some of the factors that can cause changes in 5’ deiodination (Kelly, 2000).
Selenium deficiency
Selenium deficiency effect on thyroid gland and thyroid hormone activity
If the soil is poor in selenium or there are other minerals competing for the uptake by the plant the selenium content in the plants can become insufficient. Consequently the presence of selenium in the diet will be low and can lead to selenium deficiency. Selenium deficiency occurs more often in ruminants than other species, and small ruminants like sheep and goat appear to be more susceptible. The ruminants are more disposed to selenium deficiency because they differ from other animals when it comes to the digestive system. It is believed that selenoproteins from the ingesta are generated into insoluble forms (as elemental Se) by the microorganisms of the rumen and only a part of it is integrated into bacterial proteins with the formation of amino acids. The result is a lack of Selenium in ruminants because of their use of ruminal microorganisms (Hefnawy et al, 2010).
Compared to other organs the thyroid gland contains the most selenium per gram of tissue. If the animals suffer of selenium deficiency the thyroid gland will appear pale, from beige to light brown. In post mortem examination a difference in the follicle size in animals suffering from selenium deficiency is showed. The follicles appear large and dilated, with low epithelial cells together with smaller columnar epithelial ones. The reason for this is still a question. Deficiency in selenium affect thyroid hormone metabolism through inhibiting the synthesis and activity of the iodothyronine deiodinases, which converts T4 to T3 (Kelly, 2000). As a result of the deficiency, T3 levels will decrease, while the T4 will increase. A reduction in the activity of 5` D1 will also be visible (Hefnawy et al, 2010). The most important changes in thyroid hormone metabolism may be most critical in tissues such as the brain, brown adipose tissue and the pituitary gland, which rely on the local T3 production. Selenium deficiency will also cause a decrease in the total iodine, which may lead to iodine deficiency in the thyroid and on the circulating thyroid hormones. This may further lead to hypothyroidism (Kelly, 2000). If you supplement the diet with selenium the T3 levels in the plasma has been observed to increase (Hefnawy et al, 2010).
Selenium and Thyroid diseases
The first clinical data, which establish correlation between selenium levels and thyroid metabolism, were first collected in Africa, where a high percentage of the population suffer from myxoedematous cretinism due to severe selenium and iodine deficiency. Myxoedematous cretinism is a result of persistent hypothyroidism, and if treated with iodine alone it will still persist. Further, if only treated with selenium supplement, the hypothyroidism may aggravate and lead to myxoedematous coma. From this, a link between the importance of both selenium and iodine content in the thyroid gland can be drawn, either while treating or protecting against myxoedematous cretinism (Drutel et al, 2012). Symptoms of myxoedemous cretinism are shaggy fur, massive hairloss, and swelling of the connective tissue under the skin. In humans, the myxoedema will be expressed on the face and around the eyes, where as in animals it will normally be seen around the neck (SzIE, Physiology Department et al, 2012). As mentioned earlier, iodine deficiency will lead to increased H2O2 and the selenium deficiency will lead to decrease in GPX activity. In featus’ and infants, the excess of H2O2 can’t be neutralized by the GPX, mainly leading to cell destruction and fibrosis due to macrophage activity. As a result of these processes, total destruction of thyroid gland may occur a few years, after birth. As a defence mechanism, however, hypothyroidism will increase the activity of D2 deiodinase, and will therefore make sure to maintain the local production of T3 values in the brain tissue, which is crucial for normal brain development (Drutel et al, 2012).
Experiments
To understand the importance of how selenium affect on the thyroid gland two experiments have been described below.
Experiment in mice
One mouse was fed a selenium-deficient diet and as a comparison a control mouse was fed 0.25 mg selenium/kg. They were studied 18 weeks after weaning and then the selenium content was studied in the different tissue and in the whole body (Burk et al, 2009). (Figure 4)
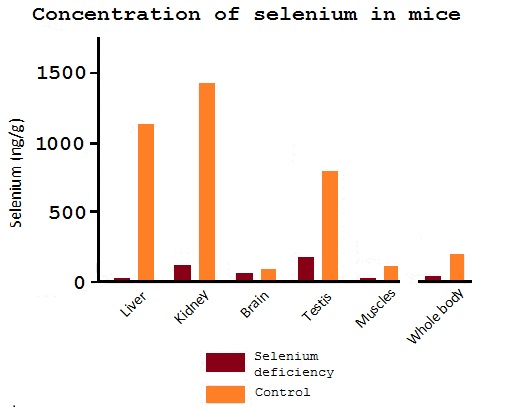
Figure 4: Results from the mice experiment (Burk et al, 2009)
Experiment: selenium influence growth via thyroid hormone status in broiler chicken
An experiment executed by He Jianhu, Akira Ohtsuka and Kunioki Hayashi concerning selenium influencing growth via thyroid hormone status in broiler chickens was published in the British Journal of Nutrition in 2000 is relevant concerning the topic of this essay. The experiments were carried out on the basis of that selenium deficiency alters the thyroid hormone synthesis in the thyroid gland and the activity of tissues specific 5’ deiodinase. Different selenium containing and selenium deficient diets were fed to chicken broilers and several observations were done; measurement of thyroid hormone, selenium content determination, hepatic type I idodothyronine deiodinase activity among others. The results of these experiments substantiate what was previously discussed; that selenium deficiency causes lower plasma T3 level and a higher level of T4 in the plasma. Consequently growth rate will decrease (Picture 5). The recorded results showed that birds consuming selenium supplemented diets (0,5 mg Se/kg) had a better growth rate than birds on selenium deficient diets in all cases. Therefore the conclusion can be drawn that dietary selenium can be supplemented to the diet and give optimal growth because it is needed to 5’-deiodenases which catalyses the conversion of T4 to T3 (Jianhua et al, 2000).

Picture 5: Result from the broiler chicken experiment (Wick et al, 2006)
Conclusion
Normal function of the thyroid gland is important for optimal metabolism. Elements, such as selenium and iodine, are essential for thyroid hormone synthesis and production. Deficiency of selenium will lead to diseases, such as hypothyroidism and myxoedematous cretinism. In most part of Europe, selenium deficiency is quite normal for domestic mammals (ruminants are more susceptible), due to the poor content of selenium in the soil. The most common treatment for selenium deficiency in ruminants, is Vitamin E and selenium. Vitamin E is mostly used for GPX deficiency treatments, while on the selenium-dependent thyroid function, selenium will most likely be the better treatment. The Vitamin E treatment is prefered above the selenium treatment, because selenium in high dosages can be toxic and lethal. Therefore,the injection treatment of selenium dependent thyroid function should be carried out carefully (Langaanes, 2012). As a better alternative, like the broiler chicken experiment points out, supplementation of selenium in the diet is more effective to both prevent deficiency and to promote normal growth and metabolism.
References
- Arthur, J.R., Nicol, F., Beckett, G.J. (1991) The role of selenium in thyroid hormone metabolism and effects of selenium deficiency on thyroid hormone and iodine metabolism. Biological trace element research, 34: 321-325 [online]
Available at http://link.springer.com/article/10.1007/BF02783686?LI=true (viewed 2012-10-15)
- Burk R.F., Hill K.E.(2009): Selenoprotein P –expression, functions and roles in mammals. Biochimica et Biophysica Acta (BBA) – General subjects, 1790:(11) 1441-1447 [online]
Available at http://www.sciencedirect.com/science/article/pii/S0304416509000786 (viewed 2012-11-01)
Christensen, K. (2006): Selenium - the Essential Trace Mineral, URL: http://www.goatbiology.com/selenium.html, viewed 2012-10-11
- Drutel, A., Archambeaud, F., Caron, P. (2012) Selenium and the thyroid gland: More good news for clinicians. Blackwell Publishing Ltd, Clinical Endocrinology 77:(6) 6-12 [online]
Available at http://onlinelibrary.wiley.com/doi/10.1111/cen.12066/abstract (viewed 2012-10-11)
- Hefnawy A.G., Tórtora-Pérez J.L.(2010): The importance of selenium and the effects of its deficiency in the animal health. Small Ruminant Research, 89:( 2-3) 185-192 [online]
Available at http://www.sciencedirect.com/science/article/pii/S0921448809003149 (viewed 2012-11-01)
- Jianhua H., Ohtsuka A., Hayashi K. (2000): Selenium influences growth via thyroid hormone status in broiler chickens. British Journal of Nutrition 84: 727-732 [online]
Available at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=884620 (viewed 2012-10-15)
- Kelly G.(2000) Peripheral Metabolism of Thyroid Hormones: A Review. Alternative Medicine Review, 5:(4) 306-333 [online]
Available at http://www.altmedrev.com/publications/5/4/306.pdf (viewed 2012-11-01)
- Langaanes, Per Erik; Norwegian Veterinarian, interviewed of Langaanes, Pernille, 2012-10-16
- Murray R.K., Granner D.K., Rodwell V.W. (2006) Harper's illustrated biochemistry, 27th edition: 243-244
- Saito, Y., Sato, N., Hirashima, M., Takebe, G., Nagasawa, S., Takahashi, K. (2004) Domain structure of bi-functional selenoprotein P. Biochem J: 841–846 [online]
Available at http://www.ncbi.nlm.nih.gov/pubmed/15117283 (viewed 2012-10-11)
- SzIE, FVM, Dept. of Physiology and Biochemistry. Physiology lecture notes (2012) Endocrinology; thyroid gland: 204-255
- Weeks, B. S., Hanna, M. S., Cooperstein, D. (2012) Diatery selenium and selenoprotein function. Med Sci Monit: 127-132 [online]
Available at http://www.medscimonit.com/fulltxt_free.php?ICID=883258 (viewed 2012-11-01)
- Whanger P.D. (2009): Selenoprotein expression and function- selenoprotein W. Biochimica et Biophysica Acta (BBA) – General subjects, 1790:(11) 1448-1452 [online]
Available at http://www.sciencedirect.com/science/article/pii/S0304416509001457 (viewed 2012-11-03)
Pictures
(All figures and pictures are prepared (redrawn) by students)
- Figure 1;
- Langaanes, P. E.(2012)
- Figure 2;
- SzIE, FVM, Dept. of Physiology and Biochemistry. Physiology lecture notes (2012) Endocrinology; thyroid gland: 204-255
- Figure 3;
- SzIE, FVM, Dept. of Physiology and Biochemistry. Physiology lecture notes (2012) Endocrinology; thyroid gland: 204-255
- Figure 4;
- Burk R.F., Hill K.E.(2009): Selenoprotein P –expression, functions and roles in mammals. Biochimica et Biophysica Acta (BBA) – General subjects, 1790:(11) 1441-1447 [online]
Available at http://www.sciencedirect.com/science/article/pii/S0304416509000786 (viewed 2012-11-01)
- Burk R.F., Hill K.E.(2009): Selenoprotein P –expression, functions and roles in mammals. Biochimica et Biophysica Acta (BBA) – General subjects, 1790:(11) 1441-1447 [online]
- Picture 5;
- Wick G., Andersson L., Hala K., Gershwin M.E., Selmi C., Erf G.F., Lamont S.J, Sgonc R. (2006) Avian models with spontaneous autoimmune diseases. Advances in immunology, 92: 71-117 [online]
Available at http://www.sciencedirect.com/science/article/pii/S0065277606920021 (viewed 2012-11-05)
- Wick G., Andersson L., Hala K., Gershwin M.E., Selmi C., Erf G.F., Lamont S.J, Sgonc R. (2006) Avian models with spontaneous autoimmune diseases. Advances in immunology, 92: 71-117 [online]