The Positive Physiological Effects of Sports Activity on the Living Organism
Contents
Introduction
Over the past few decades we have observed a substantial increase in the numbers of overweight and obese individuals in the Triad Countries, this is not limited to the human population but also affects the pets. To combat this condition that can lead to many health issues, the states promote physical activity and sports. These can participate in preventing or even reversing these conditions, with overall positive effect on health and longevity. Physical activity can be defined as any voluntary movement which leads to the uptake of energy, it can be anything from cleaning the house to working out. We are going to focus our attention on sports which is a subsection of physical activity. Sports are very different from one living organism to another, but as a general definition we can consider it as a high energy demanding activity that is practiced with the goal of improving physical condition and in many cases personal and team performances. This activity is normally practiced on a regular basis, the frequency however depends on the species practicing, the sport and the physical condition of the individual. When it comes to our pets, we have influenced their physical conditions by altering the environment they live in. By doing so we changed their natural behaviour for example horses which normally travel about 10-20km a day are kept in boxes and are worked in a more intensive way for just one hour a day. In the case of dogs who are kept in apartments without having the space to freely run at any given time but depend on their owner to give him access to larger spaces. With all of this in mind, we are going to present the physiological benefits of regular sports activity on the organism but also emphasise the problems that can occur if this is overdone. In this essay we would therefore like to further elaborate on the physiological effects of regular physical activity on the following organ systems; musculoskeletal system, nervous system, endocrine system, gastrointestinal and excretory system, immune system, cardiovascular system, respiratory system, respiratory system and the reproductive system.
In our conclusion we will give a brief summary of what are the main benefits of regular physical activity and what are the dangers associated with over-exercising. Furthermore, we would like to expand on possible methods to make our pets more active.
Skeletal Muscle
The major function of skeletal muscle is to produce motion and aid in the maintenance of posture. In order to achieve this, it must be able to efficiently adapt to the ever-changing demands exerted on it. The living organism is able to promote the latter trough training. Training can be characterised into two basic types i.e. aerobic or endurance training, which is a type of exercise that affects mainly depends primarily on the aerobic energy-generating processes in the muscle and anaerobic or resistance training, which is the type of exercise that depends primarily on the anaerobic energy-generating process in the muscle (e.g. glycolysis) (Manley, 1996).
The primary response to resistance training includes the buildup of muscle strength. On a cellular level the latter is primarily mediated by increases in the synthesis of protein filaments that constitute the actin and myosin filaments. As it has been demonstrated in human and animal studies this in turn is mediated by increased production of myogenic mRNAs, increased production of ribosomal RNA and proteins, activation of initiation factors, as well as adequate availability of appropriate amino acids (Adams, 2010; Bickel et al., 2005; Haddad and Adams, 2002).
Furthermore, it is becoming increasingly widely accepted that above mentioned processes of muscle hypertrophy (i.e. increase in muscle size), are also supported by the incorporation of new myonuclei into myofibers. The former are derived from satellite cells (i.e. the muscle stem cells responsible for longitudinal and cross-sectional postnatal growth), through a complex process of differentiation (Adams, 2010; Dayanidhi and Lieber, 2014; Petrella et al., 2008). Adaptations of muscle to resistance training also include increased size and complexity of the neuromuscular junction as well as more efficient recruitment of the muscle fibres, which further contribute to better motor performance (Birch et al., 2004; Deschenes et al., 2000; Duchateau et al., 2006). Lastly increased strength of the connective tissue in tendons, and ligaments can be observed in connection to this type of exercise (Manley, 1996; Brumitt and Cuddeford, 2015).
In endurance trained muscle an increase in size and number of mitochondria, increased capillarization of muscle, increased glycogen storage capacity as well as an increased activity of oxidative enzymes and myoglobin content, can be observed. These changes provide muscle with a better energy supply which in turn supports better performance at considerably higher rates of work . The currently accepted model suggests that on a cellular level, alterations in expression levels of diffusible gene copies (i.e. mRNAs) are responsible for modulating the above-mentioned changes (Manley, 1996; Flück, 2006).
Besides increasing athletic performance, exercise appears to be a valuable tool for disease treatment and prevention. For instance resistance exercise can help prevent and reverse muscle atrophy, that is a decrease in muscle size, which can result from aging long-term bed rest or prolonged immobilization of body parts following injury (Little and Phillips, 2009). On the other hand however, excessive demands on the muscle may result in mechanical damage to the muscle itself and this in turn may also promote mechanical damage to bones and joints, therefore training should always be progressed with caution.
Skeletal System
Bone is a very dynamic structure that is being remodelled continuously trough life of an organism in order to adapt to demands exerted on it. This remodelling is orchestrated by coordinated action of osteoblasts, osteoclasts and osteocytes. The main function of the skeletal system is to provide support for the muscle action and maintain posture (Santos et al., 2017). In response to increased load, bone tissue deforms, and the mechanosensors located on osteocytes, such as stretch-activated ion channels and integrins, change their original conformation, which will trigger a series of cellular events that will eventually lead to the deposition of new bone, by osteoblasts, as well as inhibit of bone reuptake by, at the site of deformation (Santos et al., 2017).
Due to the above-mentioned regular exercise has a positive effect on bone strength and bone mineral density which aids in the prevention of mechanical fractures and osteoporosis (a disease characterised by decreased bone mass and structural deterioration of bone tissue leading to bone fragility). On the other hand, an increased incidence of osteoarthritis (a disease characterised by degeneration of cartilage and growth of new bone around the joint), has been reported in sport participants, thus it is important to take care to avoid excessive joint stress, which is believed to be one of the major contributing factors to the condition, during exercise (Santos et al., 2017; Pereira et al., 2015).
Neural System
The neural system is a rapid response control mechanism that detects relevant information, integrates multiple sources of information and initiates appropriate action to restore homeostasis. Unfortunately, our insight into its structure and function is rather limited due to its complex structure and delicate nature, which pose a significant challenge to our research endeavors (Birch et al., 2004; McKee et al., 2014),
Nevertheless, it has been well documented that physical activity improves cognition, reduces age-related loss of brain volume, impedes neurodegeneration, improves motor function as well positively affects emotional status of the subject. Exercise promotes the aforementioned indirectly by enhancing sleep, reducing stress and promoting better general physical wellbeing of the subject as well as directly by promoting neurogenesis, cell survival, synaptogenesis, synaptic plasticity and angiogenesis. It is postulated that molecular mechanism behind the latter involve the coordinated action of several growth factors, such as brain derived neurotrophic factor (BDNF), Insulin-like growth factor 1 (IGF-1), and vascular endothelial growth factor (VEGF) (McKee et al., 2014; Baek, 2016). Current research in rats indicates that increased expression of BDNF in response to exercise is strongly correlated with increased differentiation of hippocampal adult neural stem cells into neurons and exercise-related improvement in the brain functions (Marlatt et al., 2012; Baek, 2016). A Study by Vaynman et al. even demonstrated that by blocking BDNF expression, the the enhancement of cognitive function following physical exercise can be prevented (Vaynman et al., 2004).
Although the natural hippocampal neurogenesis declines with ageing, the above described mechanisms of neurogenesis are retained over the lifespan. The latter proved of value in relieving the symptoms of Alzheimer’s and Parkinson’s disease (i.e. neurodegenerative conditions, associated with ageing) in humans and is therefore likely to positively affect analogous conditions in animals (Baek, 2016).
Immune system
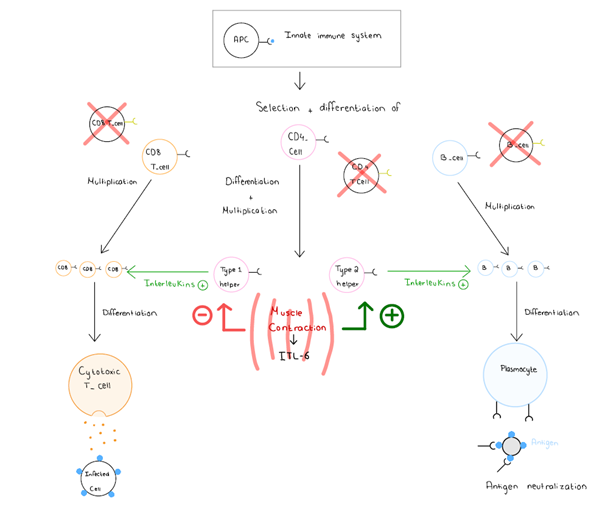
The immune system is an individual’s natural defence mechanism against infections or any nonself elements that enters the organism. This system can be subdivided into the innate and acquired immunity. The innate immune system is non specific and fast to respond to any threat and this response is characterised by inflammation. On the other hand, the acquired immune response is activated by the innate immune response. This system is specific to an antigen and takes more time to respond, but once activated it produces high levels of antibodies in the case of humoral immunity and cytotoxic-T cells in the case of cellular immunity. Whilst the effects of exercise on the immune system is a vastly investigated topic, the results are controversial depending on the intensity of the exercise considered. Karacabey et al., 2005 showed the effects of regular but moderated exercise on the organism increase the concentration of immunoglobulins between the pre and post exercise blood draws, which indicates that this kind of exercise has a positive effect on the humoral immune system. . Gleeson, 2007 further explained that during muscle contraction the muscle fibres produce Interleukin-6 (IL-6) along with other factors. IL-6 stimulates the production of type-2 T-cells which produce cytokines that stimulate the humoral response. But the same IL-6 is responsible for a reduced concentration in type 1 T-cells that produces cytokines responsible for the cellular immunity. So in the case of regular but moderate exercise IL-6 is produced which will stimulate the production of circulating antibodies and decrease the cellular immunity, which should however return to base level in 3 to 24h (see figure 1). The circulating antibodies have a relatively long lifespan, so if their production is stimulated a little at the time the circulating concentration will increase allowing a better humoral immunity. But in the case of long and strenuous activity at high frequency the production of Il-6 is much higher so there will be a much larger window when the cellular immunity is impaired leaving the organism at high risk of viral infection.
|
Figure 1 The influence of muscle contraction on the immune system, Kathleen Mulcahy, Louise Moysan, 2019. |
Reproductive system
The proper function of the reproductive system is closely related to the energy balance of the body. Therefore, it is very sensitive to small changes in food intake and energy expenditure. Physical activity can have both positive and negative effects, depending on the duration and the type of exercise.
Being overweight highly increases the risk of erectile dysfunction. In 2004, (Esposito et al., 2004) observed the change in the erectile capacity of sedentary overweight men after a training programme. Their IIEF-5 (Index of Erectile Function-5) significantly increased and some candidates even achieved a score proving that their erectile function was restored to normal range. Thus, regular physical activities associated with a proper diet would increase the chance of improving the erectile capacity.
The hypothalamic-pituitary-testicular axis regulates the reproductive system. The hypothalamic GnRH stimulates the release of FSH and LH by the pituitary gland which will promote the synthesis of testosterone (which is essential for sperm development) in the testes. Then, the increased testosterone level will have a negative feedback on the HT. It has been observed that men that participate in sports regularly have higher level of testosterone (ARI et al., 2004) and higher levels of LH and FSH than those living a sedentary life (Vaamonde et al., 2012). They seem to have a better anabolic hormonal activity and a more efficient semen production but the process by which these sexual hormones are regulated is still under discussion.
The effect of sport on the sperm quality and the spermatogenesis intensity should also be mentioned. The later decreases with aging due to an imbalance between oxidant and antioxidant compounds in the testes which leads to oxidative reactions resulting in DNA fragmentation and the death of sperm cells, both responsible for male infertility. Research shows the morphology of seminiferous tubules and the numbers of spermatozoa were significantly better in mice runners and the amounts of markers of oxidation reactions (protein carbonyls, nitrotyrosine, lipid peroxidation products) and oxidatively modified DNA were lower in their spermatogenic and Leydig cells compared to sedentary mice (Chigurupati et al., 2008). This proves that normal physical exercise reduces the risk of oxidative reaction and DNA damage in the cells of the testes. Although we must emphasize that high level physical exercise tends to have the opposite effect on oxidative reactions and sperm DNA damage (Tartibian and Maleki, 2012). The overtraining may also decrease the testosterone level below the average (Hackney, 2008).
As for women there is evidence of menstrual irregularities and abnormal ovulation in overweight individuals (Hartz et al., 1979). The most common disorder is the polycystic ovary syndrome (PCOS), where approximately 50% of affected women are overweight (Gambineri et al., 2002). PCOS develops both because of genetic and environmental factors. It causes hyperandrogenism which leads to irregular menstrual cycle and anovulation increasing the risk of infertility. Overweight women with PCOS and reproductive disorders following a weight loss programme showed improvements in their reproductive function (i.e. better menstrual cyclicity and recovery of normal ovulation) (Clark et al., 1998; Huber-Buchholz et al., 1999; Norman et al., 2004; Thomson et al., 2009). Thus, regular physical exercise associated with a specific diet helps to improve the reproductive status of overweight women but we need further studies to better understand the mechanisms that influences it. However, intensive training seems to also have a negative effect on the female reproductive system. It can induce amenorrhea (Bullen et al., 1985) which affects menstrual cyclicity, follicular development and ovulation. It originates from the disturbance of the hypothalamic GnRH control of the pituitary, resulting in an irregular LH pulse profile (Veldhuis et al., 1985; Warren, 1999).
Although numerous studies evaluating the impact of regular physical exercise on the hormones controlling the reproductive system can be found there is a lack of consensus regarding the exact mechanism and roles behind the latter. In general, it is recommended to slowly change the amount of exercise in order to allow the reproductive system to adapt to these new demands.
Excretory system
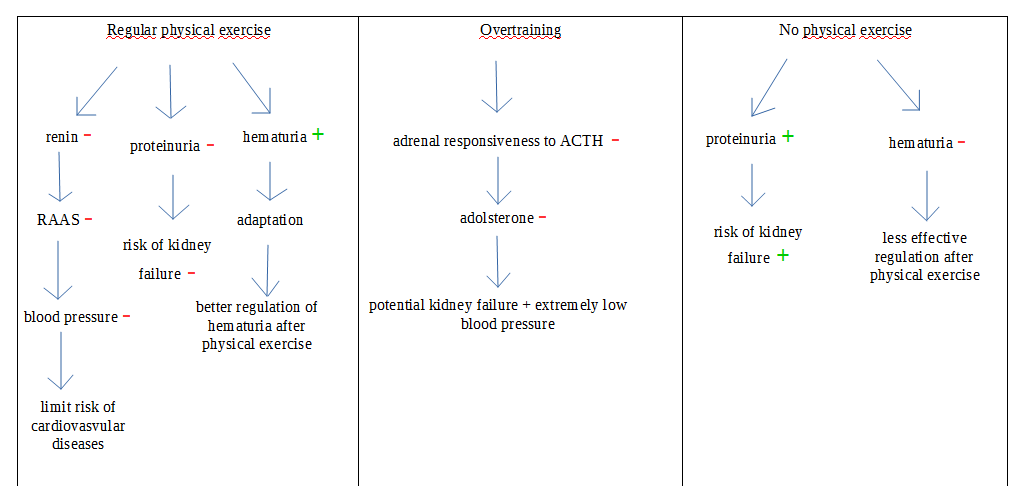
The blood pressure, the electrolyte and water balance in the kidney are controlled by the renin-angiotensin-aldosterone system (RAAS). This hormonal control is especially important during physical activity as the body loses water and minerals during sweating.
The renal function is closely linked to the blood circulation and heart function through the effect of RAAS on the blood vessels. Regular exercise training significantly decreases the effect of RAAS (Hespel et al., 1988) and significantly reduces systolic and diastolic blood pressure (Goessler et al., 2016). No relation was found between the net changes in renin concentration and changes in BP but the observed results suggest a potential role of the RAAS in the improvement of the BP after exercise training. This potential positive effect of the renal function on the circulation needs further studies to be clarified.
Nevertheless, the high level training may have different negative impacts on the bodily functions. The overtraining syndrome, a condition occurring when the body is pushed beyond its natural ability to recover, has been related to adrenal insufficiency (Ka, 2013). It can cause the adrenal hormone production to deplete. After a long period of heavy exercise training, the adrenal responsiveness to ACTH (pituitary hormone regulating the adrenal gland) is reduced. ACTH primarily acts on the release of cortisol but it may also have an effect on the release of aldosterone. The limited effect of the ACTH can result in kidney failure and hypotension.
We can also mention the presence of proteins and red blood cells in the urine after physical exercise (Bellinghieri et al., 2008). The temporary proteinuria and hematuria are normally observed during muscle work due to changes in glomerular and tubular apparatus, excessive protein synthesis, presence of free radicals, hemolysis and other mechanisms. The prevalence of proteinuria depends on the intensity of the exercise, thus high intensity exercise should be avoided if the individual has any type of kidney insufficiency. Although the protein level in urine is higher after a physical exercise, this is lower in trained than in untrained individuals. It means that regular physical training would be a solution to deal with the excessive proteinuria which can lead to kidney failure. The hematuria is more frequent in the trained than the non-trained population, although the sport-related hematuria is spontaneously resolved after the exercise while it can be a chronic problem for sedentary individuals (Bellinghieri et al., 2008). The figure below is a summary of the effect of different physical states on the organism.
|
Figure 2 The consequences of the different physical states of an individual on the excretory system, Louise Moysan, 2019. |
Digestive System
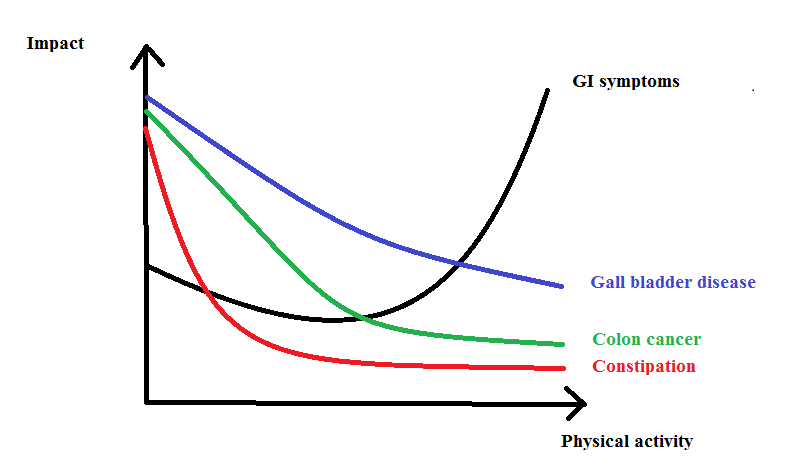
Up to now, the studies postulate that physical exercise has potential benefits on colon cancers, cholelithiasis, gastrointestinal haemorrhages (GIH) and constipation (Peters et al., 2001). Although the intensive training usually causes upper gastrointestinal (GI) issues like nausea, vomiting, abdominal pain, diarrhea and even GI bleeding (de Oliveira and Burini, 2009). These GI complications are temporary so it does not alter the health of the individual. The exact mechanisms by which the physical exercise has a positive effect on the GI tract are not yet clear.
The physically active individuals seem to be more protected from the risk of developing a colon cancer. The reduced intestinal transit time seems to limit the period of contact between the colon mucosa and the carcinogenic substances (Peters et al., 2001). Oliveria and Christos, (1997) also suggested that physical exercise has a beneficial effect in latter, because of its mood increasing action and stimulation of other organ systems (which in general improves the quality of life).
Cholelithiasis, the formation of gallstones, can be prevented by physical activity (Peters et al., 2001). Training may reduce the risk of developing gallstones by increasing the colon and gallbladder motility which avoid the cholesterol crystallization (due to biliary cholesterol secretion) (Erpecum and Berge-Henegouwen, 1999).
Another positive effect has been observed in individuals suffering from gastric and duodenal ulcer. The physical exercise contributes to the normalization of the GI microcirculation which helps healing (Efremushkin et al., 1998).
The Regular physical activity is associated with a decreased risk for severe GIH probably due to the increased GI blood flow (Peters et al., 2001).
Frequency of constipation is highly related to sedentary lifestyle and depression (Donald et al., 1985); (Kinnunen, 1991). Regular muscular exercise, even just a daily walking programme, has also a positive effect on the defecation pattern. It reduces the problem of constipation (well-formed stools, higher defecation frequency) (Schryver et al., 2005; Tantawy et al., 2017), which also stimulates good moods. The physical activity is not the only factor in the latter and it therefore needs to be always accompanied by, a proper diet and an otherwise healthy lifestyle, for maximal success. The following graph mentions the effects of the physical activity on different pathological problems.
|
Figure 3 Modified image of “Putative relationship between the incidence of some gastrointestinal diseases/symptoms and amount of physical activity, ranging from bed rest to marathon running or triathlon. The relationship of other gastrointestinal diseases (that is, diverticulosis, gastrointestinal haemorrhage, inflammatory bowel disease, peptic ulcer disease) is not depicted due to limited evidence”, Peters et al, 2001. |
Endocrine system
As mentioned previously throughout this essay sports influence many different hormones but there are a few that still need to be covered.
It has been proven that regular physical exercise decreases the level of cortisol (stress hormone). Physically active individuals deal better with the excessive amount of stress which can lead to bodily harm and negative emotions (Puterman et al., 2011).
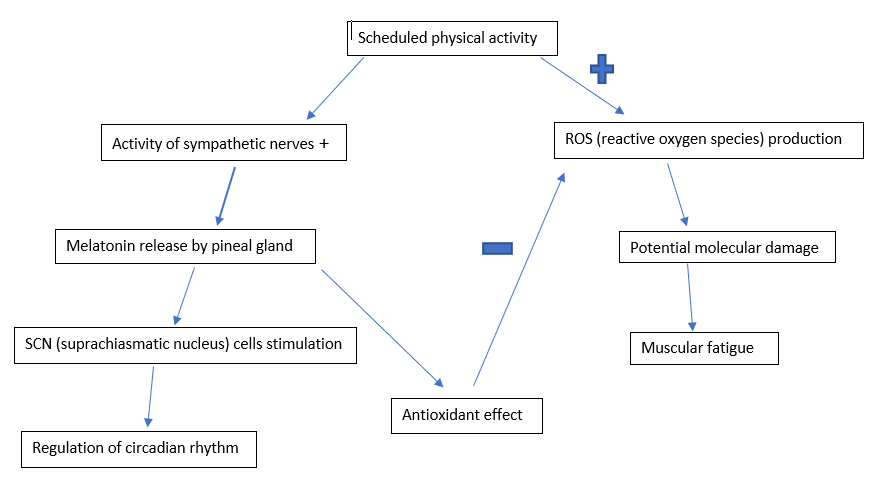
The melatonin release follows a daily (circadian) rhythm with the peak levels occurring at night. A disturbed circadian rhythm (due to jet-lag or shift-work for example) alters the sleep-wake rhythmicity. Scheduled physical activity (at the same time of the day) may act as a synchronizer of the circadian rhythm as well as aid in improving the physical performance. These benefits are clinically relevant more information is needed to further elaborate the latter (Escames et al., 2012). The following figure shows the possible explanation of the mechanism behind the above mentioned.
|
Figure 4 The mechanism explaining the reciprocal benefits due to the melatonin-exercise interaction, Louise Moysan, 2019. |
Furthermore, growth hormone (GH) is also regulated by the exercise. It has been shown that exercise stimulates normal 24-hour pulsatile GH secretion, which in turn helps to maintain normal body structure and metabolism, as well as normal blood glucose levels (Widdowson et al., 2009).
Cardiovascular system
The cardiovascular system is composed of the heart along with all arteries and veins. It contains and pumps the blood which is used as a transporter throughout the entire organism. But this system remains relatively fragile due to the thickness of certain vessels and to the constant pressure with in it. There are numerous diseases that can affect the cardiovascular system and they have many factors including sex, diet and lifestyle. Sedentary individuals are most likely to be affected by these whilst active people significantly reduce their risk (Gliemann et al., 2013).
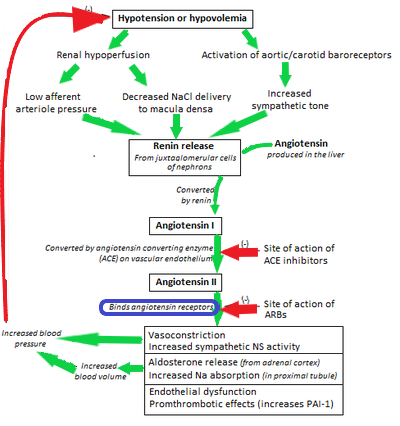
The most common diseases that are currently studied are high blood pressure and the coronary artery disease. The later is due to an increase in rigidity and thickness of the walls of certain vessels caused by a buildup of cholesterol within the tissue. This can rupture if the blood pressure increases to much which will end in the death of the patient without immediate intervention. The blood pressure however is partially controlled by the renin-angiotensin-system (see figure 5).
|
Figure 5 Modified image of “Flowchart showing the clinical effects of RAAS activity and the sites of action of ACE inhibitors and angiotensin receptor blockers”, Npatchett, 2015. |
In the case of physical activity, the expression of the angiotensin II type 1 and type 2 receptors is affected. The type 1 receptor is the most abundant in adult wile type 2 receptor is the most abundant in foetal life but very few remain in adulthood(Gao et al., 2012). During physical exercise we have observed a 77% decrease in the expression of type 1 receptor along with an increase in the expression of type 2 receptors (Adams Volker et al., 2005). Leaving the overall number of receptors (type 1+ 2) to decrease resulting in a weaker action of the vasoconstriction effect by the angiotensin, which helps to reduce the blood pressure.
It has also been observed that physical exercise increases the vasodilatory effect of acetylcholine due to the increase of nitric oxide (NO) in the endothelium (Dörnyei et al., 2000). NO has many different effects on the circulatory system but it mainely modulates the vascular tone, allowing dilatation of the vessels (Tousoulis et al., 2012).
In conclusion regular physical exercise allows the organism to reduce it’s blood pressure by limiting the effect of angiotensin and increasing the effect of acetylcholine. As for the diseases that affects directly the heart such as chronic heart failure it has been proved that a long-term regular exercise training in these individuals can significantly reduce there daily symptoms and increase their quality of life (Giannuzzi Pantaleo et al., 2003).
Respiratory system
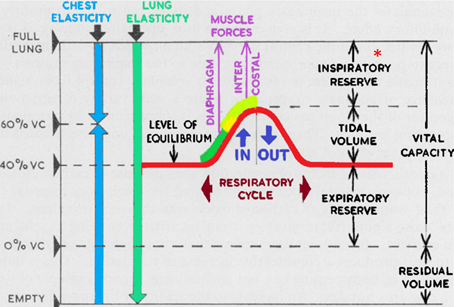
The respiratory system can be defined as series of organs that allows the gas exchange between the internal and external environment of a living organism, which takes place in the alveoli of the lungs. This is a cyclic process that involves muscle contraction for inspiration, and a passive return to the original size of stretched tissue during expiration. These repeated cycles also play a role in the carbonate buffer system keeping the pH at physiological levels. The efficiency of the respiratory system is dependent on the lung capacity which can studied thanks to the different volumes that can be observed in the following graph.
|
Figure 6 Modified image of “The mechanics of respiration”, Miles, 1969. |
Exercise has as both long- and short-term effects on this system (Miles, 1969). The most observed long-term effect in active beings is an increase in the total volume of the lung. This is particularly notable in the case of swimming and diving athletes, where an increase in volume up to 16 percent has been observed most of which is from the inspiratory reserve. This increase in volume is due to the fact that during exercise, the air no longer flows homogeneously but becomes turbulent. Which leads to a higher demand in effort from the inspiratory muscles to create a big enough vacuum, that will allow the influx of air all the way to the alveoli. This regular extra effort on the respiratory muscles encountered during exercise will cause the muscles to become stronger and more endurant ( Cf chapter on muscles). This increase in force means that in resting state they will automatically produce an increased volume of the chest cavity compared, to their previous performances. Leading to an increase in lung volume.
There are also several short-term effects that occur in response to exercise. Sports activity causes an acceleration in the metabolic processes which lead to the increase of body temperature, CO2 concentration and a decrease in pH. In response to this the living organism is going to increase ventilation to restore the balance.
Conclusion
Physical activity has many positive effects on different organ systems and thus on a living organism itself. As indicated above regular physical activity and in particularly so the practice of sport, supports the build up of muscle strength and endurance, bone mineralization, improves the immune response, supports better function of the cardiac, digestive system and respiratory systems, may aid in reproduction and normalization of the circadian rhythm as well as improves the cognitive function, reduces stress, and helps maintain normal body structure and metabolism. The latter is not only important for maintaining good health status of the individual, but can also be utilized to treat or reverse various diseases. For instance the reduction in body mass and blood pressure are important factors in improving the outcome of obesity and heart disease. Furthermore physical therapy can help patients recover from muscle dystrophy and even reverse the effects of osteoporosis. Lastly simple things like improving the GI motility with the help of light exercise can be useful in preventing severe colic episodes, which can be deadly for horses.
References
Karacabey, K., Saygin, O., Ozmerdivenli, R., Zorba, E., Godekmerdan, A., Bulut, V., 2005. A L A.
Manley, A.F., 1996. Physical Activity And Health: A Report Of The Surgeon General. DIANE Publishing.
Miles, S., 1969. The respiratory system and sport. J. R. Coll. Gen. Pract. 18, 180–185.
Pereira, D., Ramos, E., Branco, J., 2015. Osteoarthritis. Acta Med. Port. 28, 99–106.