Vasopressin antagonists
Sidsel Trygstad Bakås, Fredrik Johansen and Karijanne Kjeldsen
Vasopressin receptor antagonists are molecules designed for their inhibiting effects on the receptors for the signaling hormone vasopressin (also known as ADH). The different receptor sub-groups have different location, structure and mechanism, which can be selectively inhibited to produce or suppress a certain physiological and/or psychological effects.
Contents
1. Lecture-Specific Information
In this section we have collected some information from physiology lectures relevant to this topic.
Vasopressin, also known as antidiuretic hormone (ADH), plays the primary role in regulating the plasma osmolality. The hormone is secreted from the neurohypophysis and its secretion is primarily triggered by hyperosmosis of the plasma sensed by osmoreceptors in the hypothalamus. Stress- and pain-reactions also stimulate the secretion of ADH. Baroreceptors in the heart detecting increased blood pressure, stress and volume receptors in the left atrium and the lungs and central and atrial ANP production all have negative effects on the ADH secretion. [Kidney powerpoint]
Vasopressin stimulates the water reabsorption in the distal tubules and collecting ducts of the kidneys. It also increases the blood pressure through V1 receptors and IP3 (leading to vasoconstriction). [Endocrinology powerpoint]
Dehydration of the body leads to a decrease in blood volume. There can be different reasons to dehydration, e.g. decreased fluid intake or injury. The concentration of salts in the blood increases, which leads to a rise in osmotic pressure. The osmoreceptors in the hypothalamus respond to the change in osmotic pressure and mediate the release of ADH from the pituitary. At the same time, the sensation of thirst is triggered by the thirst center in the hypothalamus also. The result of increased ADH secretion is increased reabsorption of H2O in the kidney, which will raise the blood volume and thereby normalizing the salt concentration (and osmotic pressure).
By using an experimentally perfused kidney (heart-lung preparation) hypoosmotic urine is produced because of the lack of endocrine mechanisms. ADH will quickly readjust isosmosis. Also damage to the hypothalamic ADH secreting locus results in hyposmotic urine (e.g. diabetes insipidus) and if there is an increased diuresis due to excessive water intake, this can be promptly blocked by ADH. Hydropenia results in immediate blood-ADH increase. [Kidney powerpoint]
2. Vasopressin receptors
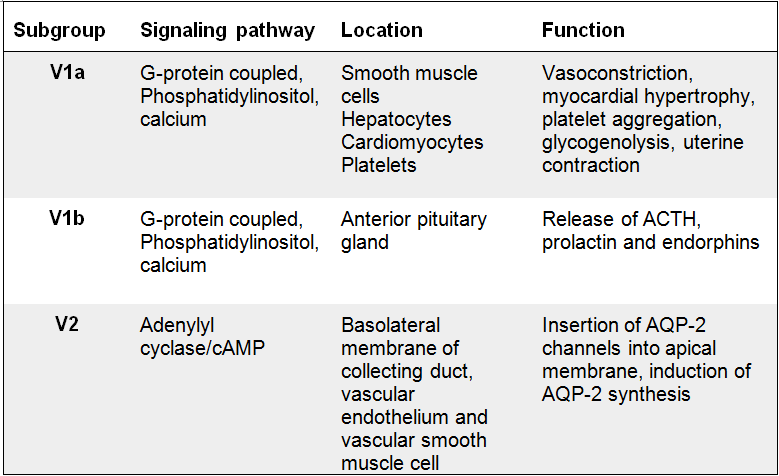
The vasopressin receptors are classified into three different subgroups which all belong to the G-protein coupled receptor family. The subgroups (V1a, V1b and V2) have different function, location and transduction mechanism. The V1a and V2b receptors are primarily related with the increase of intracellular calcium concentration, which in turn leads to vasoconstriction, while the V2 receptor is mainly associated with the antidiuretic function of vasopressin in the kidney.
V1a receptors are located in vascular smooth muscle cells, hepatocytes, platelets and cardiomyocytes. When the vasopressin is bound to the receptor, it activates phospholipases which in turn promotes hydrolysis of phosphatidylinositol biphosphate [(Birnbaumer 2000)]. The cascading effect causes a calcium outflow from the endoplasmatic reticulum. Emptying of calcium supplies triggers calcium influx via divalent cationic channels [Lemmens-Gruber and Kamyar 2006]. Increased intracellular calcium concentration initiates calcium facilitated effects such as vasoconstriction.
|
Effects of the V1a receptor are vasoconstriction, myocardial hypertrophy (thickening of the heart muscle), platelet aggregation(can lead to formation of a thrombus), glycogenolysis and uterine contraction. Vasopressin’s positive inotrope effect is caused by the binding to V1a receptors. Both the V1a and the V1b receptors have the same transduction mechanism, which commence with the hydrolysis of phospholipids. However, v1b receptors are located in the central nervous system, in cells in the anterior pituitary gland. This receptor mediates the release of ACTH, prolactin and endorphin. While the other two receptor subgroups are strictly connected to physiological effects, animal experiments links the V1b receptor to a role in emotional and psychological expression [Lemmens-Gruber and Kamyar 2006].
The V2 receptors are localized in the distal tubules and the collection duct of the kidney. The activation of the V2 receptors enables the well-known antidiuretic effect of vasopressin. When vasopressin binds to the V2 receptor, the adenylyl cyclase signaling pathway is stimulated and results in accumulation of cAMP in the cells[(Birnbaumer 2000)]. The increased intracellular cAMP concentration triggers the function of the aquaporin-2 channels which appear in the luminal side of the apical membrane of cells in the distal tubule and collecting duct in the kidney. The final result of activation of V2 receptors is reabsorption of water through the aquaporin channels.
3. Background
The first descriptions of the action mechanisms in the vasopressors and the antidiuretic activities of the pituitary extracts were at the end of the 19th- and at the beginning of the 20th centuries [Peri 2013]. It was first reported that the vasopressin rich posterior pituitary extracts increased the blood pressure, this was in 1895. 18 years later, scientists discovered that the use of these extracts actually inhibited the excretion of urine. Decades later, the osmotic and non-osmotic control systems and their interactions were discovered [Berl 2015]. It was not clear that the hormone responsible for vasopressors and antidiuretic effects was the same before after the isolation and synthesis of vasopressin [Peri 2013].
The first studies revealing that the competitive V2 receptor antagonist induced aquaresis in different animals were reported in the early 1980s. However, none of the antagonists identified seemed to be effective clinical antidiuretic antagonists. First at the discovery of the nonpeptide vasopressin type 1 (V1) receptor antagonist in 1991, fully effective vasopressin receptor antagonists were developed. This receptor antagonist counteracted vasopressin-induced vasoconstriction in rats, because the compound selectively and competitively antagonized the binding to the V1 receptor. The nonpeptide vasopressin type 2 (V2) receptor antagonist was discovered one year later, and it led to excretion of solute-free water in rats. The first successful use of a nonpeptide V2 receptor antagonist in humans was reported in 1993 [Peri 2013].
4. Peptide- and Non-Peptide Antagonists (Vaptans)
Because of the structural and interactional similarity of the three receptors, designing specific non-peptide antagonists has proven a challenge. Although vaptans are a relatively new class of drug, several specific polypeptide antagonists have been created during the last 50 years. Both types of antagonists utilize subtype-specific residues deep within the receptor binding pocket which alters the receptors ability to bind the agonist, vasopressin[Decaux, Soupart et al. 2008]. In the human V1b receptor for example, four amino acids, located in the membrane helixes, were found responsible for the binding of the antagonist[Lemmens-Gruber and Kamyar 2006].
When taken over longer time periods, the polypeptide antagonists turn agonistic in humans, making the treatment ineffective. Furthermore, the polypeptide antagonists must be administered parenteral while the non-peptide can be administered orally[Decaux, Soupart et al. 2008].
4.1. V2 receptor antagonists
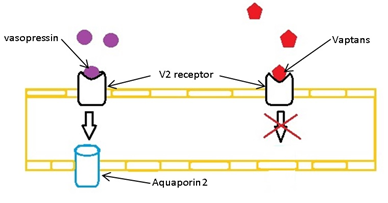
These molecules affect the V2 receptors located mainly on the renal collecting ducts. Common for all the vaptans are their mechanism of action, namely competitive antagonism. The competitive effect lowers the activation frequency of the receptor, preventing the synthesis and transport of aquaporin-2. In absence of vasopressin-stimulation, the cells are nearly impermeable to water[Solberg, O. G. and T. Omland 2007]. Accordingly, an increase in diuresis occurs.
In contrast to other diuretics such as thiazide, studies have shown a urine solute (i.e. sodium, potassium) sparing effect in the vaptans. This excretion of free water is called aquaresis. Consequently they are well suited for the treatment of euvolemic or hypervolemic hyponatremia[Decaux, Soupart et al. 2008].
|
Hyponatremia is the most frequent electrolyte disorder, a consequence of excess water relative to total body sodium and increased, normal or reduced plasma osmolality may be related. The most common form of hyponatremia encountered in clinical practice is the hypotonic hyponatremia.
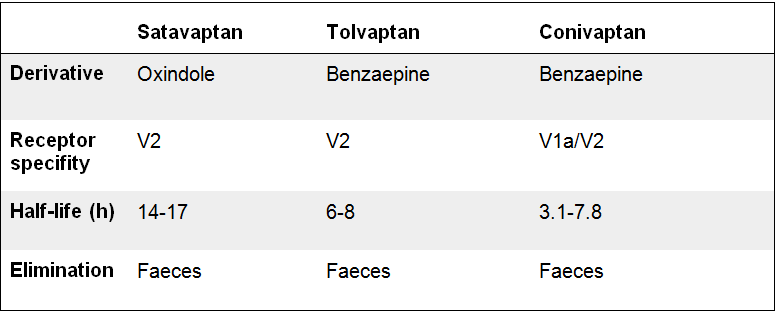
Conivaptan is an effective inhibitor of Cytochrome P450 3A4. This enzyme, found in the liver and intestines, is responsible for the metabolizing of numerous drugs and toxins. This elicits the danger of drug-drug interactions. Conivaptan demonstrates affinity to the V1a receptor as well, but no effect on pulse rate or systolic blood pressure has been attested.[Decaux, Soupart et al. 2008]
|
|
A recent study was performed in Japan examining the early use of Tolvaptan in patients with decompensated heart failure[Matsukawa, Kubota et al. 2015]. According to their hypothesis, an early administration of tolvaptan may shorten the rehabilitation time and lead to a shorter hospital stay. 102 patients with decompensated heart failure were divided into two groups; one group was administered tolvaptan within 3 days of admission, the late group later than 3 days. Both groups experienced an increase in urine-volume. The diuretic effect was significantly higher in the early group. An increase of systolic blood pressure was observed, but no notable difference between the two groups was evident. Tolvaptan furthermore produced a significant decrease in heart rate, augmented in the late group. There was no noteworthy change in glomerular filtration rate. The result was accordingly: Average hospital stay in the early group: 20.9 ± 1.3 Average hospital stay in the late group: 35.4 ± 3.7
The physiological changes above may reflect the potential benefits of tolvaptan regarding hospital rehabilitation time. Diuresis is a crucial therapy for congestive symptoms and considering the aquaretic effect of tolvaptan, the complication of hyponatremia is avoided. Regarding the importance of administration timing, it is speculated that the earlier termination in the use of carperitide(Natriuretic diuretic peptide drug commonly used in Japan) may contribute to quicker rehabilitation in the patients [Matsukawa, Kubota et al. 2015].
4.2 V1a receptor antagonists
The V1a vaptans antagonize vasopressin-mediated effects such as vasoconstriction, platelet aggregation and coronary vasospasm. Observed effects after administration of V1a antagonists are improved cardiac output, reduced mean arterial blood pressure, peripheral resistance and inhibition of myocardial protein synthesis[Decaux, Soupart et al. 2008]. Subsequently they are beneficial in the treatment of vascular diseases such as Raynaud’s disease, cerebral vasospasm and myocardial infarction. Also dysmenhorroea and preterm labour are conditions where the V1a receptor antagonist has beneficial effects.
|
In the brain, vasopressin has a vital role in regulating the social behavior and interaction, learning and memory and emotional control in mammals. Animal studies have shown that when the proper vasopressin-signaling pathway is altered, the animals develop a variety of social deficits [Carson, Garner et al. 2015]. It is also known that specific alleles of the V1A receptor gene is associated with a higher risk of autism spectrum disorders (ASD) [M. Del Valle Rubido 2015]. Although the estimated prevalence of ASD in Europe is about 0.62-0.72% [European Commission], and the number of reported cases has been increased rapidly the last 30 years, no effective pharmacotherapy is currently available.
Both animal and human studies have revealed that the V1a receptor antagonist RG7713 has a certain positive effect on core symptoms on social cognition [Meyer-Lindenberg, Kolachana et al. 2009]. However, this research is in a preliminary phase, and more studies and clinical trials are needed to discover the potential of vasopressin antagonism as a possible treatment against ASD.
4.3. V1b receptor antagonists
V1b receptors are located in the central nervous system. In the corticotroph cells of the adenohypophysis for example, they stimulate the release of ACTH. Studies performed with V1b receptor antagonist on rats have shown an inhibitory effect on corticotropin secretion, thus representing possibilities in the investigation and treatment of anxiety and stress. V1b receptors have also been characterized in neurons of the septum, cortex and hippocampus of the rat brain. Studies on these particular areas have shown an effect on various behavioral processes, particularly avoidance and defensive behavior. [Griebel, Simiand et al. 2002, Qi, Guzhva et al. 2015, Varga, Fodor et al. 2015, Bayerl, Kaczmarek et al. 2016]
As the vasopressin 1b receptor is closely related to emotional and psychological processes, recent studies are focusing on these effects. One of the latest studies, published in June 2015[Qi, Guzhva et al. 2015], were testing the hypothesis that the negative mood state associated with withdrawal to nicotine is partially caused by an increased activity of brain stress systems. This study investigated the effects of acute and chronic treatment with the Vb1 receptor antagonist SSR149415 (nelivaptan) on the dysphoria associated with nicotine withdrawal in rats. The results of this study were that the chronic treatment with the Vb1R antagonist completely prevented the brain stimulation reward thresholds associated with nicotine withdrawal. The acute treatment did only partly prevent the withdrawal to nicotine. In conclusion this study revealed that chronic treatment with Vb1R antagonists may block the negative temperament associated with smoking cessation and thereby increases the rates of relapse. [Qi, Guzhva et al. 2015]
|
Another study available from December 2015[Bayerl, Kaczmarek et al. 2016], investigated the relationship between antagonism of vasopressin 1b receptors, maternal motivation to retrieve pups, and the behavior directed towards the pups during maternal defense in lactating rats. This study was primarily designed to look at its role in specific brain sites known to be important for maternal behavior. Both the medial preoptic area (MPOA) and the medial-posterior part of the bed nucleus of the stria terminalis (mpBNST) were stimulated in the brains of virgin (control group) and lactating rats (test group). The study revealed that Vb1R blockade within the MPOA improved pup retrieval, whereas in the mpBNST, the same treatment reduced pup-directed behavior during the maternal defense test. Also, instantly after the cessation of the maternal defense test, Vb1R antagonism in both the brain regions reduced the nursing of the pups. Concluding this study, the data collected indicate that Vb1R antagonism significantly modifies different aspects of maternal behavior, but in a brain region-dependent manner [Bayerl, Kaczmarek et al. 2016].
5. Conclusion
In this paper we present an overview of the major mechanisms, effects and possibilities of vasopressin receptor antagonists. We summarize new studies and findings within the field in a simple, readable style. A major message is the diversity of functions regarding the different receptors and the effect of their antagonists. Vasopressin receptor antagonists can be considered an effective implement in the treatment of various illnesses, ranging from mental- to vascular and electrolytic disorders. Only two types are currently approved and available on the market in USA and Europe; the V2-specific Tolvaptan and Satavaptan. Henceforth more research and further clinical studies are required to unravel the full potential of the vaptans and their effects relative to other treatment options.
6. References
6.1. Articles
Bayerl, D. S., V. Kaczmarek, B. Jurek, E. H. van den Burg, I. D. Neumann, B. M. Gassner, S. M. Klampfl and O. J. Bosch (2016). "Antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats." Horm Behav 79: 18-27.
Berl, T. (2015). "Vasopressin Antagonists." N Engl J Med 373(10): 981.
Birnbaumer, M. (2000). "Vasopressin Receptors." Trends in Endocrinology & Metabolism 11(10): 406-410.
Carson, D. S., J. P. Garner, S. A. Hyde, R. A. Libove, S. W. Berquist, K. B. Hornbeak, L. P. Jackson, R. D. Sumiyoshi, C. L. Howerton, S. L. Hannah, S. Partap, J. M. Phillips, A. Y. Hardan and K. J. Parker (2015). "Arginine Vasopressin Is a Blood-Based Biomarker of Social Functioning in Children with Autism." PLoS One 10(7): e0132224.
Decaux, G., A. Soupart and G. Vassart (2008). "Non-peptide arginine-vasopressin antagonists: the vaptans." Lancet 371(9624): 1624-1632.
Griebel, G., J. Simiand, C. Serradeil-Le Gal, J. Wagnon, M. Pascal, B. Scatton, J. P. Maffrand and P. Soubrié (2002). "Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V(1b) receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders." Proc Natl Acad Sci U S A 99(9): 6370-6375.
Lemmens-Gruber, R. and M. Kamyar (2006). "Vasopressin antagonists." Cellular and Molecular Life Sciences 63(15): 1766-1779.
M. Del Valle Rubido, D. U., F. Shic, J.T McCracken, L. Scahill, O. Khwaja, L. Squassante, L. Boak, F. Bolognani, P. Fontoura, C. Wall, R. Jou, R. Loomis, M. Lyons, A. Gavaletz, J. Cowen, T. Apelian, S. Jeste, C. Ferretti, B. Taylor, G. Berlin, R. Noone, L. Antar, E. Hollander (2015). "Results from a phase I proof-of-mechanism study with a vasopressin 1A receptor antagonist in autism spectrum disorder." European Neuropsychopharmacology 25: S646-S647.
Matsukawa, R., T. Kubota, M. Okabe and Y. Yamamoto (2015). "Early use of V2 receptor antagonists is associated with a shorter hospital stay and reduction in in-hospital death in patients with decompensated heart failure." Heart Vessels.
Meyer-Lindenberg, A., B. Kolachana, B. Gold, A. Olsh, K. K. Nicodemus, V. Mattay, M. Dean and D. R. Weinberger (2009). "Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans." Mol Psychiatry 14(10): 968-975.
Peri, A. (2013). "Clinical review: the use of vaptans in clinical endocrinology." J Clin Endocrinol Metab 98(4): 1321-1332.
Qi, X., L. Guzhva, Y. Ji and A. W. Bruijnzeel (2015). "Chronic treatment with the vasopressin 1b receptor antagonist SSR149415 prevents the dysphoria associated with nicotine withdrawal in rats." Behav Brain Res 292: 259-265.
Solberg, O. G. and T. Omland (2007). "Vasopressinantagonisme – nytt behandlingsprinsipp ved hjertesvikt." Tidsskrift for Den norske legeforening 2(127): 179-182.
Directorate-General for Health and Food Safety. (2014). "Autistic Spectrum Disorders (ASD)." Accessed April 2016, from http://ec.europa.eu/health/major_chronic_diseases/diseases/autistic/index_en.htm.
6.2. Figures
Figure 1: Fredrik Johansen, Microsoft Paint (Apr. 2016)
Figure 2: National Center for Biotechnology Information. PubChem Compound Database; CID=158348, https://pubchem.ncbi.nlm.nih.gov/compound/158348 (accessed Apr. 17, 2016), National Center for Biotechnology Information. PubChem Compound Database; CID=216237, https://pubchem.ncbi.nlm.nih.gov/compound/216237 (accessed Apr. 17, 2016), National Center for Biotechnology Information. PubChem Compound Database; CID=151171, https://pubchem.ncbi.nlm.nih.gov/compound/151171 (accessed Apr. 17, 2016).
Figure 3: National Center for Biotechnology Information. PubChem Compound Database; CID=60943, https://pubchem.ncbi.nlm.nih.gov/compound/60943 (accessed Apr. 17, 2016).
Figure 4: National Center for Biotechnology Information. PubChem Compound Database; CID=9895468, https://pubchem.ncbi.nlm.nih.gov/compound/9895468 (accessed Apr. 17, 2016).
6.3. Lecture notes
Veterinary physiology, Szent Istvan University; Physiology of the kidney (p. 112 and 132), http://www.vetphysiol.hu/eng/lecturenotes.php. Accessed in the academic year 2015/2016.
Veterinary physiology, Szent Istvan University; Endocrinology (p. 99), http://www.vetphysiol.hu/eng/lecturenotes.php. Accessed in the academic year 2015/2016.