West Nile Virus and its effect on the kidney
By Christel Stokke, Kine Eggan, Anja Sveen
Contents
General Introduction
Fig.1. West Nile Virus
The West Nile Virus is a single-stranded RNA virus in the family Flaviviridae, genus Flavivirus.(Gubler., et al 2007). And is related to the pathogens responsible for dengue fever and yellow fever. (Lindenbach., et al 2011) The West Nile Virus was dicovered in Uganda in 1937, and has been responsible for thousands of cases of mortality and morbidity in humans, horses and birds. Epidemics were localized to Europe, Africa, the Middle East, and parts of Asia, and primarily caused a mild febrile illness in humans. In the late 1990's the virus became more vicious and expanded its geographical range to North America. (Hayes., et al 2001) During the late summer of 1999, individuals where diagnosed in New York State, and in 2000, the epizootic expanded to 12 states. Today the West Nile Virus can be found in many avian and mosquito species throughout North America. Data shows that from 1999 to 2010, more than 2.5 million people were infected and there were more than 12,000 reported cases of encephalitis or meningitis and also more than 1,300 deaths.
Transmission cycle
The West Nile Virus is transmitted by mosquitoes which are the prime vector, with birds, being the commonly infected animal and serving as a prime reservoir host. The mosquitoes become infected when they bite and feed on infected birds. One or two weeks later, the infected mosquito can transmit the virus to another animal or human. Usually the virus cycle is between mosquitos and birds, but there are a lot of incidental hosts, like humans and horses. However, humans, horses and other animals are ‘dead end’ hosts. This means that they do not develop high levels of the virus in their bloodstream, and therefore cannot pass the virus on to other biting mosquitoes. Some additional routes of human infection have also been documented, but in very small number of cases. Like transmission through blood transfusion, organ transplants, exposure in laboratory settings, breastfeeding and even during pregnancy from mother to baby. The West Nile Virus is not transmitted from person-to-person or from animal-to-person or from handling live or dead infected birds.
Fig.2. Transmission cycle of the West Nile Virus (Sveen A. 2013)
When infected with the West Nile Virus, signs of illness may not always be detected in people and animals. Approximately 80% of West Nile Virus infections in humans are subclinical and this is very similar to horses where most infections are asymptomatic. In humans, West Nile fever (WNF), which occurs in 20 percent of cases, is a febrile syndrome that causes flu-like symptoms, like fever, headaches, fatigue and muscle pain. Of all the people infected with West Nile Virus, 70-80% do not even develop any symptoms. Out of the symptoms that do appear, febrile illness is the most common one. Other symptoms that may occur is vomiting, diarrhea, headache, joint pains, body aches, or rash. Except weakness and fatigue, people infected with this type of WNV will in most cases recover completely. However there are some severe symptoms also. This includes a neurologic illness, such as encephalitis or meningitis. The illness can be known as West Nile encephalitis. This illness concerns the inflammation of the central nervous system, which is caused by the infection of the WNV. Less than 1 % of the people infected will get theses diseases. Signs of headache, neck stiffness, high fever, disorientation, coma, tremors, seizures or paralysis, can detect neurologic illness. Those people that are at greater risk for serious illness are the ones suffering from different types of medical conditions, such as diabetes, kidney diseases and hypertension. Unfortunately, some of the neurological effects may be permanent, but recovery after several weeks or months is possible. 10 percent of people suffering from neurological infection due to West Nile Virus will die.
Symptoms in horses
As mentioned before, the West Nile Virus does not always lead to symptoms of illness in animals or people. Horses however, seem to be more receptive to infection with the virus. In horses, as in human, the virus infects the central nervous system, through a neurological disease known as encephalitis. Primary symptom in the horse is loss of appetite and depression. In addition, several other symptoms such as fever, weakness and paralysis of hind limbs, ataxia, seizures, walking in circles or worst-case coma, may occur. Having these symptoms does not necessarily mean that the horse have West Nile encephalitis. Certain other diseases can cause a horse to show similar symptoms.
Treatment
Currently, patients infected with West Nile Virus have limited treatment options. Supportive care is the primary course of action. There is no FDA-licensed vaccine to fight the West Nile disease in humans, despite the research of many laboratories and institutions and vaccines available for use in horses.
Studies have suggested that ribavirin and interferon alfa-2b may be useful in the treatment of West Nile virus disease, but the efficacy of these agents has not been demonstrated in controlled clinical trials. An FDA-approved clinical trial has been launched to study the effectiveness of interferon alfa-2b in patients with West Nile encephalitis. To date, no controlled trials have studied the use of corticosteroids, anticonvulsants, or osmotic agents in the treatment of neurologic disease caused by West Nile virus.
Prevention
Prevention In humans, no vaccine is licensed for the prevention of WNV disease, although labarotury development is currently underway. Thus mosquito-control programs and personal protective measures are important in preventing WNV infection. In horse, on the other hand, specific vaccines are available. West Nile virus vaccines are produced either as an aid in prevention or reduction of viremia, encephalitis and clinical diseasea.
Global distribution
Phylogenetic studies have identified 2 main lineages of WNV strains. In the early 2000-s strains from lineage1 were present in Africa, India, and Australia and were reponsible for outbreaks in Europe, in the Mediterranean Basin, and in North America, whereas lineage 2 strains had been reported only in sub-Saharan Africa and Madagascar. The Hungarian equine WNV outbreak reported in 2008 was the first to be caused by a lineage 2 sub-Saharan strain in Europe. The pathogenicity of this lineage 2 strain resembled that of lineage 1 strains, and its sudden spread was unpredictable.
The WNV is found in Africa, west and central Asia, The Mediterranean, Middle East, and Europe.(Hayes., et al 1989). A large epidemic of WNV infection occurred in Bucharest, Romania in 1996, where 835 patients were hospitalized. Out of the infected number, 393 patients tested positive for WNV antibodies. (Tsai.,et al 1998). The virus later caused a large epidemic in Volograd, Russia (Platonov., et al 2001), and afterwards jumping the Atlantic, causing the epidemic in Queens, New York.(Nash., et al 2001). This epidemic was recognized in late August 1999, when an unusual cluster of elderly patients showed signs of viral encephalitis. (Asnis., et al 2000). The virus extended its range throughout much of the eastern parts of the USA during 1999-2002. The virus reached California in 2002 and 2003, where it caused the largest epidemics of arboviral meningoencephalitis in the history of the country.(Hayes., et al 2006).
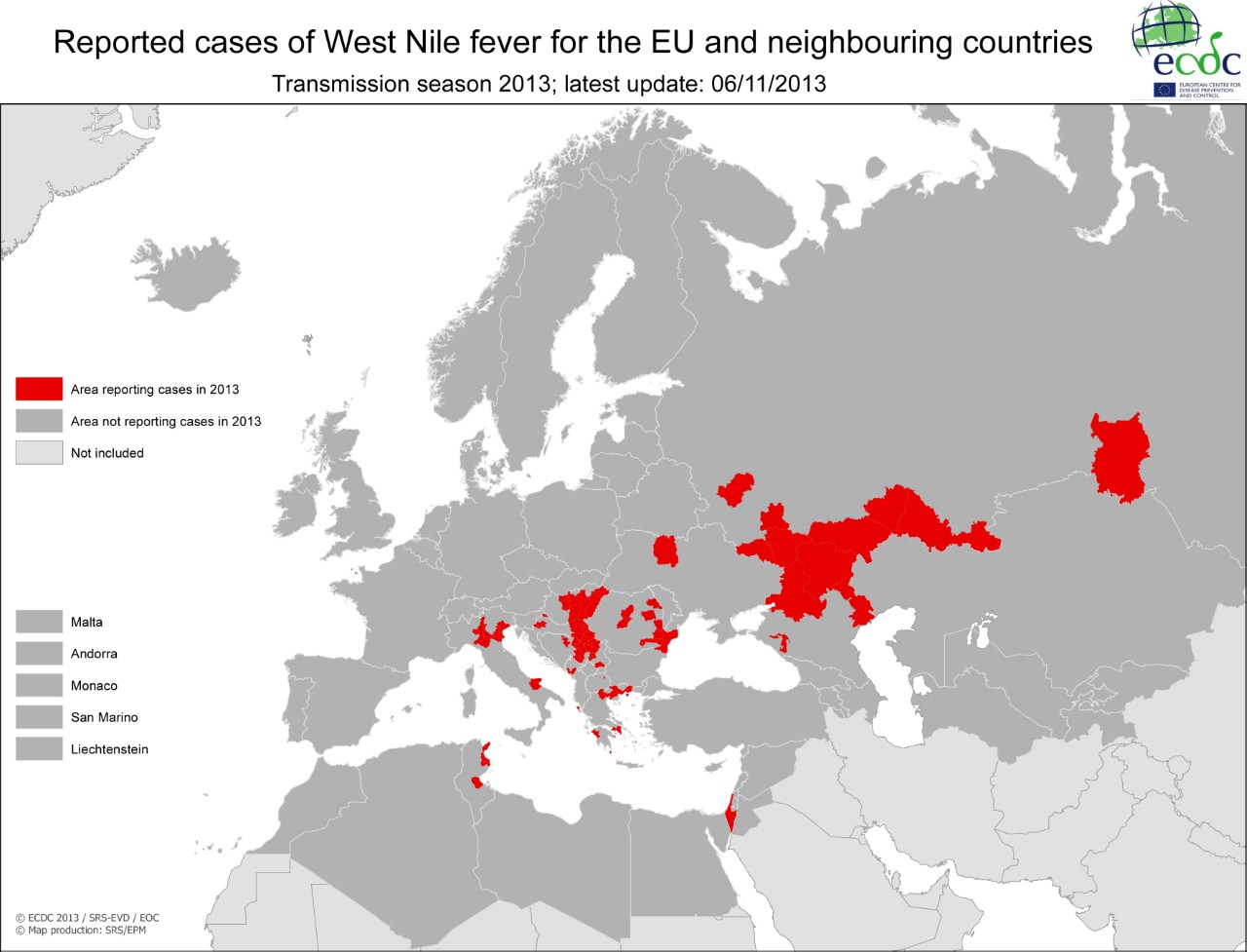
Fig.3. Global transmission of WNV 2013
Humans
If we focus on the United States, no WNV cases had been reported prior to 1999. During the 1999 encephalitis outbreak, mentioned earlier, there were 62 diagnosed human cases and out of them seven deaths.
• In 2000: 21 diagnosed human cases and two deaths.
• In 2001: 66 diagnosed cases and nine deaths.
• In 2002: 4161 diagnosed cases and 277 deaths across the United States
• In 2003: 9175 human cases reported
Horses
• In 1999: approximately 25 horses became ill from infection with West Nile Virus.
• In 2000: 60 documented clinical cases of infection. Approximately 60% of horses that actually showed signs of illness in 1999 and 2000 recovered from the infection. Others were euthanized or died as a result of infection. Many more horses were infected without showing any clinical symptoms of disease.
• In 2001: 159 documented clinical cases of infection.
Kidney
The kidney is involved in maintenance of Homeostasis (isovolemia, isosmosis, isoionia, isohydria) Also storage of essential substances like water, electrolytes, glucose, and aminoacids It also participates in acid-base balance, cardiovascular regulation (especially through Angiotensin II synthesis) It takes part in elimination of exogenous and endogenous organic components (protein-metabolic endproducts, toxins, medicines.) Last but not least it is involved in hormone production (direct or indirect) 1-25-OH-D3; erythropoetin; PGE2; T3 (from T4 converted by kidney deiodase).
GFR and Creatinine Clearence
Glomerular Filtration Rate
The volume of fluid filtered per minute from the glomerular capillaries into Bowman`s capsule is called the glomerular filtration rate (GFR). The GFR is the product of the net filtration pressure across the glomerular filter and the filtration coefficient (Kf) GFR = Kf x net filtration pressure The filtration coefficient (Kf) is the product of the fluid permeability of the glomerular filter and the area available for filtration. (Sjaastad.,et al 2010)
The Glomerular Filtration Rate can be described easier as the rate of blood flow through the kidneys. GFR can vary depending on age, sex, and size. The measurement of GFR can’t be executed directly. The measurement of creatinine and creatinine clearance is used instead.
Creatinine
During normal breakdown of muscle tissue, a waste product called creatinine is produced continuously. It is filtered from the blood through the kidneys and excreted in urine. Almost none of it is reabsorbed. The blood creatinine level is measured to test the kidney function.
Creatinine Clearance
The amount of blood the kidneys can make free of creatinine for each minute is called the creatinine clearance.
Creatinine Clearance Rate
The kidneys' ability to handle creatinine is called the creatinine clearance rate, which helps to estimate the glomerular filtration rate. In general the creatinine clearance is a good estimation of the glomerular filtration rate. Testing the rate of creatinine clearance shows the kidneys' ability to filter the blood. As renal function declines, creatinine clearance also goes down.
Two main ways we can use creatinine tests to measure kidney function
1. By collecting a sample of urine, creatinine clearance can be precisely determined.
2. Using a single blood level of creatinine, GFR can be estimated.
The higher the blood creatinine level, the lower the estimated GFR and creatinine clearance. The 24-hour urine collection test for creatinine clearance is used in a smaller degree than the blood test estimation method for GFR. Kidney disease is demonstrated by a low GFR or creatinine clearance. The decline in kidney function can be either acute or chronic. Acute decline is often sudden and often reversible, and chronic is long-termed and in most cases irreversible. To identify if the kidney disease is acute or chronic, repeated GFR or creatinine clearance measurements over time can be executed.
Effect on the kidney
Today, studies are being done on the effect of WNV on the kidney. As we have mentioned earlier, the disease doesn’t have to show any symptoms but inflammation on the brain, fatigue and paralysis can occur. Researches are now investigating the fact that even a mild infection of the WNS, may lead to kidney disease. However, it is not yet determined if there is a link between the two.
On one hand, according to an epidemiologist, Kristy Murray, a researcher at Baylor College of Medicine in Houston, the WNV may have a negative effect on the kidney. Her research started in Texas 2009, when a man infected with the virus, announced that he also had kidney disease. He died within a year. Murray then collected 25 samples of survivors of WNV, and she found that five of the samples contained viral RNA. By detecting viral RNA in the urine, this suggested that the virus had established itself in the kidney.(Murray., et al 2010).By investigating excess protein in the urine, the indication of long-term kidney disease could be carried out. She did this to examine if the virus would harm the kidneys over time. Out on 139 samples collected, Murray reported that 40% of the group showed signs of long-term kidney disease.(Nolan., et al 2012). On the other hand, there is a skeptical response to her studies. Lyle Peterson, director of the division of vector-borne infectious diseases, sees no cause for alarm given the current evidence. He argues with Murray’s studies, and points out that it did not include a control group. He also shows to studies done in Colorado, where no viral RNA where found in the urine collected. (Gibney., et al 2011).
But according to Murray, there is an art in detecting RNA in the urine. The single-stranded fragments are easily broken apart by enzymes in the fluid. With the help of enzymes, the single stranded fragments can easily be broken apart in the fluid. The samples can also be destroyed when freezed or thawed during shipping and storing. The samples that only traveled one hour from Murray’s lab to the Univeristy of Texas Medical Branch for independent testing, confirmed her findings.(Murray., et al 2010). She hopes and is determined to get the evidence to prove the skeptical wrong. And with a new lab and a four-year grant from the National Institutes of Health, she plans to recruit 440 people, half with WNV, to a study, to look for kidney disease. Hopefully we will know soon if there is link between the virus and kidney disease. Frederick Murphy, a virologist at the UTMB (Univeristy of Texas Medical Branch) states that if the problem were known, it would be easier to monitor the West Nile Patients. As soon as enzymatic abnormalities of the kidney are detected, it would be easier to figure out a way to prevent the progression of further kidney disease.
Summary
We first heard about the West Nile Virus after visiting Ullö, a large animal clicic for horses. After doing some research on the virus, we found that it is relevant in todays society, so we decided to explore the virus more and see the effect it had on the kidney. We got in contact with Dr Orsolya Kutasi, a vetrinarian at Ullö clinic. She is currently investigating the WNV and the effect it has on the kidney in horses. So far she has tested the urine of 5 horses with acute West Nile Virus infection. And in all 5, proteinuria was not detected, which indicates a negative result not linking the West Nile Virus to have an effect on the kidney.
As a summary, we can conclude that nothing is yet determined, we can only speculate if kidney disease can be linked to the West Nile Virus.
List of references
Asnis DS., Conetta R., Texiera AA. (2000). The West Nile virus outbreak of 1999 in New York: the flushing Hospital experience. 30:413-8.
Bakonyia T., Ferenczic E., Erdélyid K., Kutasie O., Csörgőf T., Seidelg B., Weissenböckh H., Bruggeri K., Bánc E., Nowotnya N. (2013); Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe; Veterinary Microbiology. 165; 61-70.
Colpitts T.M., Conway M.J., Fikrig E. (2012); West Nile Virus; Biology, transmission and human infection. Clinical Microbiology Reviews. 25(4); 635-648.
Cushing M.M., Brat D.J., Mosunjac M.I., Hennigar R.A., Jernigan D.B., Lancotti R., Petersen L.B., Goldsmith C., Rollin P.E, Shieh W., Guarner J., & Zaki S.R. (2004); Fatal West Nile Virus encephalitis in a renal transplant recipient. American Journal of Clinical Pathology. 121; 26-31.
Department of Physiology and Biochemistry; Physiology of the kidney. Szent István University, Faculty of Veterinary Science.
Gibney, K. B., Lanciotti, R. S., Sejvar J. J., Nugent, T. C., Linnen, J. M., Delorey, M. J., Lehman, J. A., Boswell, E. N (2011). West Nile Virus RNA Not detected in urine of 40 people tester 6 years after acute West Nile virus Disease. 203, 344–347.
Gubler D.J. (2007); The continuing spread of West Nile Virus in the Western Hemisphere; Clinical Infectious Diseases. 45(8): 1039-1046.
Gubler DJ., Kuno G., Markoff L. (2007). Flaviviruses In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; p. 1153-252.
Hayes C. (1989). The arboviruses: epidemiology and ecology. CRC Press; 59-88.
Hayes CG. (2001) West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci. 2001;951:25–37.
Hayes EB., Gubler DJ. (2006) epidemiology and clinical features of an emerging epidemic in the United States. 57:181-94.
Huhn G.D., Sejvar J.J., Montgomery S.P., & Dworkin M.S. (2003); West Nile Virus in the United States; An update on an emerging infectious disease; American Family Physician. 15;68(4): 653-661.
Lindenbach BD., Rice CM. (2001) Flaviviridae: the viruses and their replication. In: Knipe HP, editor. Fields Virology. Lippincott, Williams, & Wilkins; Philadelphia: pp. 991–1041.
Maxman A. (2012), The hidden treat of West Nile Virus - researchers probe possible link with kidney disease; Nature. 489, 349-350.
Murray K., Walker C., Herrington E., Lewis JA., McCormick J. (2010). Persistent infection with West Nile virus years after initial infection. J Infect Dis. 201(1): 2–4.
Murray KO., Nolan MS., Yan C. (2012) Persistence of Immunoglobulin-M antibodies up to 8 years following infection with West Nile virus. Clin Vaccine Immunol Submitted. e40374
Nash D., Mostashari F., Fine A. (2001). The outbreak of West Nile virus infection in the New York City area in 1999. Med;344:1807-14.
Rossi S.L., Ross T.M., & Evans J.D. (2010); West Nile Virus; Clinics in laboratory medicine. 30(1); 47-65.
Sjaastad V.O, Sand O., Hove K. (2010); Physiology of Domestic Animals. 473-474.
Tsai TF., Popovici F., Cernescu C. (1998). West Nile encephalitis in southeastern Romania. Lancet; 352:767-71
Websites
http://www.cdc.gov/westnile/transmission/ (CDC - centers for disease control and prevention)
http://www.westnile.state.pa.us/animals/horses.htm (Pennsylvania`s West Nile Virus Control Program)
http://www.ecdc.europa.eu/en/healthtopics/west_nile_fever/pages/index.aspx (European Centre for Disease Prevention and Control)
http://www.webmd.com/a-to-z-guides/creatinine-and-creatinine-clearance-blood-tests (WebMD)
Pictures
Figure 1: http://en.wikipedia.org/wiki/File:Em_wnvirus_j7908i.jpg
Figure 2: Drawn by Sveen Anja (2013)
Figure 3: http://www.ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/pages/index.aspx
