Contents
Contents
Endocrine Disrupting Effects of ZEA
At an introductory level, Yang et al, (2015) state that endocrine disruptors are chemicals that interfere with the hormone systems and produce adverse developmental, reproductive, neurological, and immunological effects in mammals. Endocrine disruptors can be found in many products including plastic bottles, metal food cans, detergents, flame retardants, food, toys, cosmetics, and pesticides.
Endocrine disruptors are chemicals, known as EDC’s, that can interfere with endocrine systems at certain doses. These disruptions can cause cancerous tumours, birth defects, and other developmental disorders. Any system in the body controlled by hormones can be derailed by hormone disruptors. An endocrine disruptor affects hormonal actions in different ways for example they may directly influence hormone biosynthesis, metabolism, transport and mechanism of action on both the receptor and post-receptor levels.
An endocrine disrupting chemical (EDC) represents a large range of agents from natural compounds such as hormones, plants and fungal constituents. Some EDCs act like "hormone mimics" and trick the body into thinking that they are hormones , while some block natural hormones from doing their job. ZEA is a prime example of a hormone mimic. Other EDCs can increase or decrease the levels of hormones in our blood by affecting how they are made, broken down, or stored in our body. And others can change how sensitive our bodies are to different hormones.
One such EDC is zearalenone or ZEA, a potent estrogenic metabolite.
Effects of ZEA
|
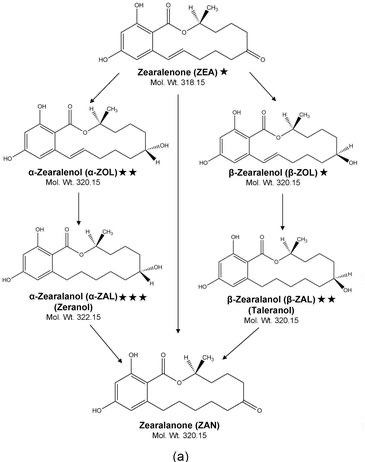
Figure 1 ZEA and its derivatives (Kleinova et al, 2002) |
According to Minervini et al, (2001), zearalenone (ZEA) and its derivatives (alpha- zearalenol known as A-ZOL, beta-zearalenol known as B-ZOL, zeranol known as A-ZAL, taleranol known as B-ZAL and finally zearalenone known as ZAN), see Figure 1.
The precise part of zea being degraded is the c-8 keto group according Olsen et al, (1981).These derivatives also play an important part in the endocrine disrupting effect of ZEA in particular zeranol.
Utermark and Karlovsky, (2007) further state that zearalenone (ZEA) and its derivatives exert estrogenic and anabolic effects on mammals. Carryover of zearalenone from infected grain to feedstuff causes reproductive problems in pigs, sheep, and other farm animals, including precocious sexual development, vulva enlargement, pseudopregnancy, loss of embryos, and reduced litter size.
Keller et al, (2015) state that zearalenone (ZEA) and its derivatives are mycotoxins with estrogenic effects on mammals. The biotransformation for ZEA in animals involves the formation of two major derivatives , α- and β-zearalenol (α-ZOL and β-ZOL), which are subsequently conjugated with glucuronic acid. For the most part the long lasting known effects of ZEA can be categorised into three main areas; those effects that alter metabolic balance; those effects that contribute to developmental disorders and those effects that negatively impact the reproductive cycle. In fact the greatest effects of ZEA can be found in the reproductive system.
Effects on the Reproductive System
ZEA has the ability to cause adverse reproductive effects due to its ability in mimicking the hormone estradiol. Wang et al, (2014) declared zearalenone resembles 17beta-estradiol an endogenous estrogen in both its structure and shape which is clearly the prime reason for its estrogenic activity as it activates the estrogen gene to cause the effects.
ZEA causes numerous toxic effects in both domestic and laboratory animals, the most common of which relates to the reproductive system. Zearalenone once administered can be most commonly found in the reproductive organs and reproductive system. It is observed in the female reproductive tissue (ovary and uterus) as well as the adipose tissue and interstitial cells of the male reproductive organ: the testes as recently proven by Kuiper-Goodman et al, (1987).
It effects many animals such as swine, equine & bovine, which we explore below. Swine are the most susceptible species to the reproductive effects of Zearalenone as discovered by many such as Gareis et al, (1990), Gajecki et al, (2002), Andretta et al, (2008).
Reproductive effects in the swine species
Swine are the most susceptible species to Zearalenone’s endocrine disrupting effects, with prepubertal animals (gilts) the most sensitive. As such it has heavily affected the pork industry. It has been reported Vanyi et al, (1993); Katzenellenbogen and Korach, (1997); Obremski et al, (2003) and Malekinejad et al, (2007) that pigs are more sensitive to the reproductive effects of ZEA than other domestic animals. ZEA has an effect on weaned gilts causing vulva hypertrophy and ovarian atrophy. According to Obremski et al, (2003), ZEA causes sterility in sows by inciting ovarian disorders where Oocytes die in follicles and therefore ovulation cannot occur. ZEA, very similar to 17 beta-E2 inhibits the secretion of steroid hormones, thus in turn would disrupt oestrogen responses , also suppressing the maturation of ovarian follicle (Katzenellenbogen and Korach, 1997).
In a study by Malekinejad et al, (2007), gilts were given a diet that contained 3.61 to 4.3 mg/kg ZEA from puberty to mating stages. In about 45% of these gilts, pseudo pregnancies were induced with ZEA. Along with this, ZEA had a suppressing effect on the pig oocyte progression through meiosis by inducing the malformation of meiosis spindles.
In their study, Vanyi et al, (1993) concluded hyperestrogenism is a common effect of ZEA in porcine, especially new born piglets. Typical clinical signs of this would be swelling of the vulva, increase in uterine size and secretions, mammary gland hyperplasia and secretion and prolonged oestrus due to the estrogenic activity seen to occur on behalf of zea being present. Secondary complications are still births and vaginal and or rectal prolapse indirect effects of the zea activity.
Kanora and Maes, (2009) observed that pregnant sows and gilts receiving food that is contaminated with ZEA would show smaller litters. This would have a more significant effect if given at early pregnancy as ZEA interferes with the response of the endometrium to progesterone during the embryo implantation.
Reproductive effects in equine species
In the equine species there is limited information and experimental evidence to date on the effects of dietary exposure to ZEA on reproduction. Zearalenone is absorbed by the gastrointestinal tract quickly after it is ingested. According to Olsen et al, (1987) its effects then occur due to the interaction of the mycotoxin with the enzyme 3 alpha (beta) - hydroxysteroid dehydrogenase, that is responsible for metabolising it into its derivatives.
In one of a number of experiments carried out by Minervini et al, (2006), they investigated the effect of ZEA in equine species. The study on granulosa cells in vitro, collected from mare ovaries during the breeding season concluded that ZEA could be the reason for reproductive failures according to the cellular disturbance in vitro. Minervini et al, (2006) go on to state that it (ZEA) can also effectively stimulate follicular atresia.
However, a more recent experiment carried out by Vance et al, (2019) would seem to contradict Minervini et al, (2006). This experiment involved the splitting of mares into three groups, each of which were administered a certain dose of ZEA which continued for three oestrus cycles. The effects were monitored, and the results collected showed that ZEA does not have much influence on reproduction as it did not cause any reproductive effects. However, Vance et al, (2019) qualified this further by stating ‘when consumed in low amounts. Juhasz et al, (2001) supported Vance et al (2019) theory of no effects being witnessed on reproductive parameters in equine species, when they investigated the effect of a low dosage of ZEA. Here they proved that treatment did not affect the duration of follicular phase in mares.
In the case of equine we found that there is not sufficient evidence to prove nor eliminate the effects of ZEA on the reproductive cycle in horse so therefore more research must be done to confirm the effects.
Reproductive effects in bovine species
As is the case with equine, the research & results related to the effects of ZEA on reproduction in the bovine species are very conflicting.
There are a few cases that support the fact that Zearalenone does not cause any reproductive effects in the bovine species. In their studies, Weavers et al, (1986a) verified that Zearalenone in the bovine species failed to produce any obvious effects on reproduction but did note that the conception rate was decreased by about 25% on account of ZEA’s estrogenic avctivity. They also showed no effect on differential cell counts, clinical signs and sexual behaviour, therefore according to this scientific study, imposing no threat to dairy cow health. Although the above case has proven no significant effects on the reproduction system occurs, Weaver et al, (1986b) cited a common problem in that the corpus lutea were smaller in bovine administered with Zearalenone as indicated through rectal palpation. Estradiol has a primitive role in reproduction and sexual function as well as an impact on health of other tissues, the decrease in size of the corpus lutea occurs due to mimicking effect of ZEA .
However, in the particular case of dairy cows, more promising and positive results are being proven.
Notwithstanding Weavers et al, (1986a & 1986b) experiments, the effects of ZEA were seen in a later experiment by Towers et al, (1995) where high levels of Zearalenone in the blood seemed to result in low fertility. Towers et al, (1995) also discovered symptoms of the ZEA toxicosis that included mammary enlargement of virgin heifers.
Coppock et al, (1990) carried out an experiment that concluded that diets with about 660 ppb ZEA resulted in poor consumption, depressed milk production, increased reproductive tract infections and total reproductive failure, therefore contradicting the earlier studies of Weaver et al, (1986a & 1986b).
Having reflected on a number theories regarding the effects of ZEA on the reproductive system in swine, equine and bovine species we have found that in the bovine species (particularly in the case of dairy cows) the effects are more prominent than the equine. However, it was evident that the swine species are still the most dominant species affected by Zearalenone. It is also very clear that the reproductive effects and all the symptoms mentioned above in each species happen on account of ZEA’s ability to impersonate the hormone estradiol therefore proving to be an endocrine disruptor due to its relationship with the endcorine system through the hormone estradiol.
Effect on Fetal life
|
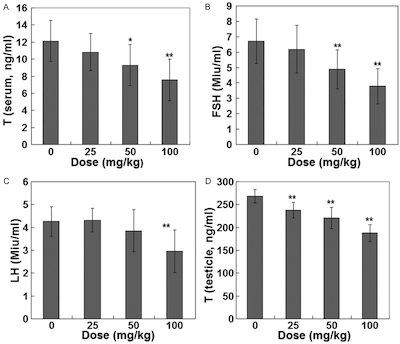
Figure 2 ZEA Effect on hormone level (Bo et al, 2015) |
In domestic animals ZEA plays an important role in placenta and fetal life. The mammalian placenta acts as a selective barrier. Its effectiveness depends on the number of layers between fetal and maternal blood, but ultimately it determines what enters and leaves the fetal bloodstream, and therefore dictates the influence of ZEA on fetal life. This placenta crossing of the mycotoxin was discovered in experiments carried out on rats and mice by Bernhoft et al, (2001). This the in-turn effects the litter size and the foetus. In the case of mice, Perez – Martinez et al, (1996) reported that alpha-ZOL (a derivative of ZEA) administered to pregnant mice caused anomalies in fetal testicular development. This impact of ZEA on testicular development is further supported by Bo et al, (2015) in his experiment to investigate the effects of Zeranol on spermatogenesis and sex hormone levels of male mice.
In the experiment, the mice were mock-treated or treated with Zeranol 25, 50 or 100 mg/kg for 25 days. The levels of serum/testicular testosterone, Follicle Stimulating Hormone(FSH) and serum luteinizing hormone(LH) were determined using radioimmunoassay. The results showed that Zeranol could decrease the levels of serum Testosterone, FSH and LH as well as testicular testosterone in male mice see figure 2. It was concluded that Zeranol could interfere with sex hormone levels by effecting the hypothalamic-pituitary-testicular axis. This hypothalamic-pituitary-testicular axis plays a key role in regulation of spermatogenesis, . FSH and LH secreted by the anterior pituitary in response to gonadotropin-releasing hormone (GnRH) released by the hypothalamus, together with testosterone (T) secreted by the testicles play important roles in sexual function and reproductive function. The effects of this endocrine disruptors ZEA with estrogenic activity on the male reproductive system could lead to male infertility, decline in male fertility, etc.
The work of Perez-Martinez et al, (1996) the more recent work of Bo et al, (2015) suggests that ZEA was able to pass through the placenta and potentially affect the fetal testicular function as well as fertility in male species this could be hypothesized to have the same effect on humans but has not been investiated to date.
Effect as Growth Promoter in Animals
Zeranol, also known as zearanol, α-zearalanol, or simply zearalanol, a derivative of zearalenone was approved 40 years ago by the U.S. Federal Drug Administration (FDA) as an anabolic growth promoter for use in the U.S. beef industry to improve feed efficiency, weight gain, meat-to-fat ratio and carcass quality (Zhong et al, 2011).
According to Song and Choi, (2001), zeranol implantation improved average daily weight gain, feed conversion, dressing percentage and yield grade of cattle, as well as increasing dry matter intake. According to Grigsby and Trenkel, (1986), this growth promotion can be attributed to the estrogenic effects of zeranol which increase growth rates through an indirect action on the pituitary gland that cause the increased release of somatotropin. It also showed excessive increase area such as carcass weight and backfat percentage.
Overall zeranol is proved to enhance growth rates of cattle production course, with the exception of the suckling and pre-weaning period, and therefore growth performance has been seen to changeable. However, the safety of using zeranol as a growth promoter has been debated for many years. Its positive effect as a growth promoter in animals also has a negative effect on the human population that consumed meat (mostly bovine) that had been treated with this growth promoter. The conclusion of many the studies (Lin et al, 1996; Swan et al, 2007; Ye et al, 2009) was that there is high risk of the development of breast cancer through the ingestion of meat treated with growth promotors such as zeranol. Lin, (2001) in his PhD supports this view finding a putative link between ZEA and risk for human breast cancer growth as mentioned below in Carcinogenic Effects of ZEA
As a result it was banned by the European union in 1981 as the carcinogenic effect (also discussed above) was evident in the human population that consumed the beef treated with this growth promoter. However other countries still used zeranol as a growth promoter, so further actions were taken and in 1989 the European Union prohibited the import of beef products from the United States or Canada that use zeranol as a growth promoter (Updike et al, 2007).
The use of ZEA as a growth promoter and its causative effect on the advanced thelarche can also be linked to problems in puberty in many species, including humans. Refer to Effects on Puberty in Humans.
Carcinogenic Effects
In both humans and animals a particular effect of Zearalenone is the threat of carcinogenicity. Some of the most common cancers are hormone-dependent and in the case of ZEA, it has been proven to cause various types of cancers such as liver, kidney, oesophageal, breast, lung and stomach, as well as cancers of the reproductive organ cancer.
In terms of endocrine disrupting carcinogenic effects, and according to Kowalska et al, (2016), ZEA mainly effects the pituitary and gonad functions. Many research studies have reported its role in breast cancer development and progression, where ZEA caused a proliferation-stimulating effect on the estrogen-sensitive cell lines T47D and MCF-7 when administered in concentration ranges similar to those of naturally occurring estrogens (Khosrokhavar et al, 2009; Dees et al, 1997; Ahamed et al, 2001; Ahamed et al, 2002).
Pfohl-Leszkowic et al, (1995) showed the presence of DNA adducts responsible for starting cancerous cells in female mice. Schothorst and Van Egmond, (2004) supported Pfohl-Leszkowic et al, (1995) they discovered that exposure to ZEA in mice can cause hepatocellular ademos and pituitary hormones resulting in reproductive organ cancer. They proposed that the mycotoxin ZEA, as an endocrine disruptor, focused its supressing effect on pituitary and gonadal function and therefore could contribute to hormone dependent cancers in both humans and animals.
This carcinogenic effect of ZEA was further supported by Bhatnager et al, (2002) when they proved that both endometrial hyperplasia, as well as cervical cancer are caused by ZEA.
In their study on a cohort of young girls, Clavel-Chapelon et al, (2002) found that these girls were especially vulnerable to the health effects of the mycotoxin, finding that early puberty can be linked to later life breast cancer.
Lefers et al, (2001) found that in vitro, zeranol stimulates growth and replication of human breast tumor cells that are sensitive to estrogen at rates similar to those induced by the natural hormone estradiol and the known carcinogen diethylstilbestrol (DES). This was supported by Liu and Lin, (2004) who found evidence that zeranol is comparable to the natural hormone, estradiol, in its ability to transform normal human breast cells into cells showing signs of early cancer development.
In a later study by Khrosrokhavar et al, (2009), while it was concluded that low concentrations of ZEA is needed for processes of the breast tissue, they also suggested a higher level of ZEA intake can proliferate certain breast cancer cells therefore triggering carcinogenesis.
Effects on Puberty in Humans
There is an evident link between the development of central precocious puberty (CPP) and the causative agent zearalenone. CPP is a condition that occurs when puberty starts before the age of 8. In various regions around the world different cases of both premature thelarche and CPP have been reported. CPP and thelarche occur due to the miminking effect of the endocrine disruptor on the hormone estradiol. As this hormone estradiol is responsible for the development of secondary sexual characteristics such as breasts it might suggest the link to therlache as an effect of ZEA which has been proven below by various people.. As for ZEA causing CPP, CPP can be traced back to the pituitary gland and the sex hormones, Schothorst and Van Egmond, (2004) and Pfohl-Leszkowic et al, (1995) as previously stated proposed that ZEA focuses its effects on the pituitary and gonadal function therefore proposing the link between CPP and ZEA.
For example, Fara et al, (1979) identified a school in Italy with reported cases of children showing signs of thelarche. Italy was a region at the time consuming a lot of meat in which their production involved the growth promoter Zeranol. In Puerto Rico, Comas, (1982); Saenz de Rodriguez, (1984); Saenz de Rodriguez et al, (1985); and Larriuz‐ Serrano et al, (2001), discovered a large epidemic of the same effects and did various research to prove that ZEA, used as growth promoter in dairy and meat production there, was in fact the reason for puberty and breast effects. Similarly in their study, Szuets et al, (1997) also discovered thlearche in Hungary where they proved a high concentration of ZEA in approximately 14% (5/36) of the tested early thelache patients.
More recently, and again in Italy, Massart et al, (2008), carried out experiments on children found with CPP and determined that ZEA was present in a few, further solidifying the previously suspected theories of ZEA having an impact on the development of CPP in children. Although concentrations were determined in Massarts studies, it was to a much lesser extent as previously suggested by Szuets et al, (1997).
Furthermore Massart et al, (2008), determined that patients who tested positive for ZEA had a higher growth rate than those who did not display any traces of ZEA . This piece of experimental evidence supports Grigsby and Trenkel, (1986) who discovered growth promotion can be attributed to the estrogenic effects of zeranol which increase growth rates through an indirect action on the pituitary gland that cause the increased release of somatotropin. Refer to Growth promoter as previously discussed above.
Effect on Immune Function
According to a study carried out by Hueza et al, (2014), they found a reduction in body weight gain in rats that were administered ZEA. This reduction in body weight was not fully explained by diminished food consumption. With respect to acquired and innate immune responses, no statistically significant differences in delayed-type hypersensitivity were noticed; however, in the ZEA-treated rats, antibody production and peroxide release by macrophages were impaired. Hueza et al, (2014) suggested that the observed results could be related to ZEA activity on estrogen receptors. Estrogens are known to have inhibitory effects on body weight gain in animal models, thus, the pronounced decrease in the body weight of ZEA-treated rats observed in this study may be a direct effect of the estrogenic property of the mycotoxin.
Hueza et al, (2014), verified that similar to estrogen, ZEA can modulate most aspects of immune responses and impair lymphoid organs, resulting in thymus atrophy; the mycotoxin can alter thymus and spleen lymphocyte phenotypes and decrease peroxide production by peritoneal macrophages; and unlike estrogen, ZEA can impair the T cell-dependent humoral immune response.
Wang et al, (2018) supports Hueza et al, (2014) in their study of the effects of Zea on intestinal microflora and intestinal mucosal immunity in mice. Their study showed that ZEA not only caused inflammation of the mucous membrane but also disturbed the microecological balance of the intestine in mice. This drives the damage to the morphological structure of the intestinal mucosa. Thus, Wang et al, (2018) believe that ZEA may be a potential factor in inducing inflammatory diseases in the human intestinal tract.
Other Known effects
Effect on Hormone Production
Frizzell et al, (2011) also referred to cortisol suggesting that ZEA and both ZOLs (α-Zearalenol and β-Zearalenol) might modulate the production of progesterone, testosterone, cortisol and estradiol in human adrenocortical carcinoma H295R cells. The modulation is caused at the level of nuclear receptor signalling and alters the hormone production.
Indirect Effect on Intestinal Tract
In recent studies, the brain-gut axis of such has been thoroughly looked at, it has been shown that interrelationships are present between the hypothalamic–pituitary–adrenal (HPA) axis, the enteric nervous system and the gut-associated immune tissues under stress. Parameters of the HPA axis include the corticotropin-releasing hormone (CRH). The hypothalamic paraventricular nucleus secretes such. The anterior pituitary secretes adrenocorticotrophic hormone (ACTH). The zona fasciculata of the adrenal cortex secretes cortisol (Sugaya et al, 2015)
In the case of cortisol, Wan et al, (2016) showed that mycotoxins, such as ZEA, can cause changes in the composition in the mucin monosaccharides of chicken intestines, thus affecting the mucin function. This effect of ZEA on mucin production suggests that an indirect effect on the cortisol production from the adrenal cortex can occur, as cortisol is a stress hormone released when there is disruption or dysfunction caused on a biological system as seen here, on the intestinal tract. This statement is supported by Rodino-Janeiro, (2015) who said that the intestinal barrier dysfunction caused the release of CRH therefore effecting the cortisol release.
Conclusion
Zearalenone (ZEA) is a mycotoxin which is classified as a xenoestrogen, an exogenous compound which resembles the structure of naturally occurring estrogens with its chemical structure. It is mainly produced by Fusarium fungi and commonly found in food and feed in the temperate climate zone. Growth occurs more often in storage than in the field.
It causes many adverse effects on mammals, such as in the reproductive system, the immune function, growth promotion, a variety of cancers as well as indirect effects such as the intestinal mucosa. Our review of some of current academic material has clearly identified Swine as being especially susceptible to Zearalenone effects.
In humans this can lead to disorders of the hormonal balance of the body, which in consequence may lead to numerous diseases of reproductive system such as prostate, ovarian, cervical or breast cancers. So the importance of monitoring ZEA concentration in food and identifying its potential effect on our health is becoming more and more apparent. Its impact on our reproductive health and the ever-daunting threat of carcinogenesis highlight the necessity of further investigation into Zealerone influence on human health. It was clear from the literature that we reviewed that there is much academic material on the endocrine disrupting effects of Zea on the animal world but limited literature on the effects on humans.
In conclusion. whether it be in animals or in humans, Zea is clearing a disruptor of the endocrine system and therefore caution should be taken in its application.
References
Ahamed, S.; Foster, J.S.; Bukovsky, A.; Wimalasena J. (2001): Signal transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Journal of Molecular Carcinogolgy. 30: (2) 88-98
Ahamed. S.; Foster, J.S.; Bukovsky, A.; Diehl, J.A,; Wimalasena J. (2002): Removal of Cdk inhibitors through both sequestration and downregulation in zearalenone-treated MCF-7 breast cancer cells. Journal of Molecular Carcinogology 34: (1) 45-58
Andretta, I.; Lovatto, P.A.; Hauschild, L.; Dilkin, P.; Garcia, G.G.; Lanferdini, E.; Cavazini, N.C.; Mallmann, C.A. (2008): Feeding of pre-pubertal gilts with diets containing zearalenone. Journal of Arquivo de Zootecnia 60: (229) 1227–1233.
Bernhoft, A.; Behrens,G.H.; Ingebrigtsen, K.; Langseth, W.; Berndt, S.; Haugen, T.B.; Grotmol, T. (2001): Placental transfer of the estrogenic mycotoxin zearalenone in rats. Journal of Reproductive Toxicology 15: (5) 545-550
Bhatnager, D.Yu.J.; Ehrlich, K. C. (2002): Fungal allergy and pathogenicity. Journal of Chemical Immunology 81: 167–206.
Bo, C.; Zhao, W.; Jia, Q.; Yang, Z.; Sai, L.; Zhang, F.; Du, Z.; Yu, G.; Xie, L.; Zhang, Z. (2015): Effects of α-zearalanol on spermatogenesis and sex hormone levels of male mice. International journal of clinical and experimental medicine, 8: (11) 20002–20013.
Bouhet, S.; Oswald, I.P. (2005): The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Journal of Veterinary Immunology Immunopathology 108: (1-2) 199–209.
Comas, A. P. (1982): Precocious sexual development in Puerto Rico. Journal of the The Lancet 1: (8294) 1299–1300.
Coppock, R.W.; Mostrom, M.S.; Sparling, C.G.; Jacobsen, B.; Ross, C. (1990): Apparent zearalenone intoxication in a dairy herd from feeding spoiled acid-treated corn. Journal of Veternary Human Toxicology 32: (3) 246-248.
Clavel-Chapelon, F., (2002). Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Journal of Br J Cancer 86: (5) 723–727.
Dees, C.; Foster, J.S.; Ahamed, S.; Wimalasena, J. (1997): Dietary estrogens stimulate human breast cells to enter the cell cycle, Environ. Journal of Health Perspect 105: (Suppl 3) 633-636
Fara, G. M.; Del Corvo, G.; Bernuzzi, S.; Bigatello, A.; Di Pietro, C.; Scaglioni, S.; Chiumello, G. (1979): Epidemic of breast enlargement in an Italian school. Journal of The Lancet 2: (8137) 295–297.
Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sorlie, M. (2011): Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Journal of Toxicology Letters 206: (2) 210-217
Gajecki, M. (2002): Zearalenone undesirable substances in feed. Polish Journal of Veterinary Sciences, 5: (2) 117–122.
Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. (1990): Cleavage of zearalenone-glycoside, a “masked” mycotoxin during digestion in swine. Journal of Veterinary Medicine Series B, 37: (1-10) 236–240.
Grigsby, M.E.; Trenkle, A. (1986): Plasma growth hormone, insulin, glucocorticoids and thyroid hormones in large, medium and small breeds of steers with and without an estradiol implant. Journal of Domestic Animal Endocrinology 3: (4) 261-267
Hueza, I.M.; Raspantini, P.C.; Raspantini, L.E.; Latorre, A.O.; Górniak, S.L. (2014): Zearalenone, an Estrogenic Mycotoxin, Is an Immunotoxic Compound. Journals of Toxins (Basel) 6: (3) 1080–1095.
Juhasz, J.; Nagy, P.; Kulcsar, M.; Szigeti, G.; Reiczigel, J.; Huszenicza, G. (2001): Effect of low-dose zearalenone exposure on luteal function, follicular activity and uterine oedema in cycling mares. Journal of Acta Vet. Hung 49: (2) 211–222.
Kanora, A.; Maes, D. (2009): The role of mycotoxins in pig reproduction : a review. Journal of VETERINARNI MEDICINA, 54: (12) 565–576.
Katzenellenbogen, B. S.; Korach, K. S. (1997): A new actor in the estrogen receptor drama–enter ER-beta. Journal of Endocrinology 138: (3) 861–862.
Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C. A.; Cavaglieri, L.; Venâncio, A. (2015): Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Journal of Toxins 7: (8) 3297–3308.
Khosrokhavar, R.; Rahimifard, N.; Shoeibi, S.; Hamedani, M. P.; Hosseini, M.J. (2009): Effects of zearalenone and α-Zearalenol in comparison with Raloxifene on T47D cells. Journal of Toxicology Mechanisms and Methods 19: (3) 246-250
Kleinova, M.; Zollner, P.; Kahlbacher, H.; Hochsteiner, W.; Lindner, W. (2002): Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. Journal of Agricultural & Food Chemistry 50: (17) 4769–4776.
Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. (2016): Zearalenone as an endocrine disruptor in humans. Journal of Environmental Toxicology and Pharmacology 48: 141-149
Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. (1987): Risk assessment of the mycotoxin zearalenone. Journal of Regulatory Toxicology and Pharmacology 7: (3) 253-306.
Larriuz‐ Serrano, M. C.; Perez‐ Cardona, C. M.; Ramos‐Valencia, G.; Bourdony, C. J. (2001): Natural history and incidence of premature thelarche in Puerto Rican girls aged 6 months to 8 years diagnosed between 1990 and 1995. Journal of Puerto Rico Health Sciences 20: (1) 13–18.
Leffers, H.; Naesby, M.; Vendelbo, B.; Skakkebeak, N.E.; Jorgensen, M. (2001): Oestrogenic potencies of zeranol, oestradiol, diethylstilbestrol, bisphenol-A and genistein: Implications for exposure assessment of potential endocrine disrupters. Journal of Human Reproduction 16: (5) 1037-1045.
Lin, Y.C.; Mulla, Z.; Kulp, S.K.; Sugimoto, Y.; Farrar, W.B.; Brueggemeier, R.W. (1996): Biological activity in serum and meat of Zeranol implanted beef cattle: Regulation of proliferation and estrogen-induced gene expression in normal breast cells and MCF-7 cells. Journal of Proc 10th Int Cong Endocrinol 1: 792-798
Lin, Y.C. (2004): Transformation of MCF-10A human breast epithelial cells by zeranol and estradiol-17B. Journal of Breast J, 10: (6) 514-521.
Malekinejad, H.; Schoevers, E. J.; Daemen, I. J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J.; et al. (2007): Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Journal of Biological Reproduction 77: (5) 840–847.
Massart, F.; Meucci, V.; Saggese, G.; Soldani, G. (2008): High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. Journal of Pediatrics 152: (5) 690–695.
Minervini, F.; Giannoccaro, A.; Fornelli, F. et al. (2006): Influence of mycotoxin zearalenone and its derivatives (alpha and beta zearalenol) on apoptosis and proliferation of cultured granulosa cells from equine ovaries. Journal of Reproductive Biology Endocrinology 30: (4) 62
Obremski, K.; Gajecki, M.; Zwierzchowski, W.; Zielonka, L.; Otrocka-Domagala, I.; Rotkiewicz, T. (2003): Influence of zearalenone on reproductive system cell proliferation in gilts. Journal of Veterinary Science 6: (4) 239–245.
Olsen, M.; Pettersson, H.; Sandholm, K.; Visconti, A.; Kiessling, K.H. (1987): Metabolism of zearalenone in sow intestinal mucosa in vitro. Journal of Food Chemical Toxicology 29: (9) 681-683.
Pérez-Martínez, M.J.; García-Iglesias, M.C.; Ferreras-Estrada, A.M.; Bravo-Moral, J.; Espinosa-Alvarez, A. (1996): Escudero-DíezEffects of in-utero exposure to zeranol or diethylstilboestrol on morphological development of the fetal testis in mice. Journal of compartment pathology 114: (4) 407-418
Pfohl-Leszkowicz, A.; Chekir-Ghedira, L.; and Bacha, H. (1995): Genotoxicity of zearalenone, an estrogenic mycotoxin: DNA adduct formation in female mouse tissues. Journal of Carcinogenesis 16: (10) 2315–2320.
Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; Di Pasquale, E.; Perrier, J.; Oswald, I.; Maresca, M. (2015): Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Journal of Molecular Nutrition and Food Research 59: (6) 1076–1087.
Rodiño-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. (2015): Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. Journal of Neurogastroenterol Motility 21: (1) 33-50.
Saenz de Rodriguez, C. A. (1984): Environmental hormone contamination in Puerto Rico. Journal of Medicine 310: (26) 1741–1742.
Saenz de Rodriguez, C. A.; Bongiovanni, A. M.; Conde de Borrego, L. (1985): An epidemic of precocious development in Puerto Rican children. Journal of Pediatrics 107: (3) 393–396.
Schothorst, R.C.; Van Egmond, H.P. (2004): Report from SCOOP task 3.2.10 ‘collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states’. Subtask: trichothecenes. Subtask: Trichothecenes. Journal of Toxicology Letters 153: (1) 133–143.
Song, M.K.; Choi, S.H. (2001): Growth Promoters and Their Effects on Beef Production. Asian-Australasian Journal of Animal Science 14: (1) 123-135.
Sugaya, N.; Izawa, S.; Saito, K.; Shirotsuki, K.; Nomura, S.; Shimada, H. (2015): Effect of prolonged stress on the adrenal hormones of individuals with irritable bowel syndrome. Journal of Biophyscosocial Medicine 9: (1) 4
Swan, S.H.; Liu, F.; Overstreet, J.W.; Brazil, C.; Skakkebaek, N.E. (2007): Semen quality of fertile US males in relation to their mothers' beef consumption during pregnancy. Journal of Human Reproduction 22: (6) 1497-1502.
Szuets, P.; Mesterhazy, A.; Falkay, G. Y.; Bartok, T. (1997): Early thelarche symptoms in children and their relations to zearalenon contamination in foodstuffs. Journal of Cereal Research Communications 25: (3) 429–436.
Towers, N.R.; Sprosen, J.M.; Webber, W. (1995): Zearalenone metabolites in cycling and non-cycling cows. Journal of Toxinology and Food Safety: 46-47
Ueno, Y.; Kubota, K. (1976): DNA-attacking ability of carcinogenic mycotoxins in recombination-deficient mutant cells of Bacillus subtilis. Journal of Cancer Research 36: (2 Pt 1) 445–451.
Updike, M.; Sawdy, J.; Wang, L.; Liu, S.; Huang, Y.; Ye, W.; Wick, M. (2007): Primary cultured human breast epithelial cells up-regulate protein disulfide isomerase in response to zeranol. Journal of Anticancer Research 27: (1A) 407–410.
Vance, C. K.; King, E. H.; Bowers, S. D.; Ryan, P. L.; Walters, K.; Shappell, N. W. (2019): Reproductive Performance of Mares Fed Dietary Zearalenone. Journal of Frontiers in veterinary science 6: 423.
Vanyi, A.; Bata, A.; Glavits, R.; Kovacs, F. (1993): Perinatal oestrogen syndrome in swine. Journal of Acta Vet Hung 42: (4) 433–446
Wan, M.; Turner, P.; Allen, K.; Nezami, H. (2016): Lactobacillus rhamnosus GG modulates intestinal mucosal barrier and inflammation in mice following combined dietary exposure to deoxynivalenol and zearalenone. Journal of Functional Foods. 22: 34–43.
Wang, X.; Yu, H.; Shan, A.; Jin, Y.; Fang, H.; Zhao, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; Wang, J.; Zhang, J. (2018): Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Journal of Food and Agricultural Immunology, 29: (1) 1002-1011,
Weaver, G.A.; Kurtz, H.J.; Behrens, J.C.; Robison, T.S.; Seguin, B.E.; Bates, F.Y.; and C.J. Mirocha, C.J. (1986a): Effect of zearalenone on the fertility of virgin dairy heifers.. Journal of Veterinary Research 47: (6) 1395-1397.
Weaver, G.A.; Kurtz, H.J.; Behrens, J.C.; Robison, T.S.; Seguin, B.E.; Bates, F.Y.; and C.J. Mirocha, C.J.. (1986b): Effect of zearalenone on dairy cows. Journal of Veterinary Research 47: (8) 1826-1828.
Ye, W.; Xu, P.; Threlfall, W. (2009): Zeranol enhances the proliferation of pre-adipocytes in beef heifers. Journal of Anticancer Research 29: (12) 5045-5052.
Zhong, S.; Ye, W.; Feng, E.; Lin, S.; Liu, J.; Leong, J.; Ma, C.; Lin, Y.C. (2011): Serum Derived from Zeranol-implanted ACI Rats Promotes the Growth of Human Breast Cancer Cells In Vitro. Journal of Anticancer Res 31: (2) 481-486
Figures
Figure 1 Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. Journal of Agricultural & Food Chemistry from Kleinova et al, (2002)
Figure 2 Effects of α-zearalanol on spermatogenesis and sex hormone levels of male mice. International journal of clinical and experimental medicine, from Bo et al, (2015)
Supporting Information
The Endocrine System
The endocrine system is the collection of glands that produce hormones that regulate metabolism, growth and development, tissue function, sexual function, reproduction, sleep, and mood, among other things. The endocrine system enables the transport of hormones produced by endocrine glands (pituitary gland, thyroid gland, parathyroid glands, adrenal glands, pancreas, ovaries (in females) and testicles (in males)) via the bloodstream to the target organs.
ZEA
What is it?
According to Zhang, et al (2018) ZEA is a non-steroidal estrogenic mycotoxin produced by many types of fusarium species with fusarium graminearum being the most important and the main producer of this endocrine disruptor.
How is it produced?
ZEA develops in the conditions that present high humidity and alternating temperatures. Its formation can be witnessed in temperate regions. In particular, conditions are often seen during the autumn harvest with the warm days (20 degree Celsius) and the cold nights (7 degree Celsius). Zhang, et al (2018) go on to say that “conditions with a large amount of moisture are particularly important in the generation of ZEA”.
What is its origin?
ZEA most often appears in livestock grains worldwide. It cannot be found in meat or animal products such as dairy as the conditions necessary for ZEA production (see above) are not provided therefore it can’t develop. Livestock grains such as wheat, maize, barley, corn and oats contain ZEA. Maize takes up a major portion of total grain crop worldwide and is seen to be the most susceptible grain to ZEA development. Again, according to Zhang, et al (2018) the fusarium fungi responsible for the production of ZEA are present on almost all continents and have the ability to infest both pre and post harvested livestock grain mentioned above.
What is it structure?
ZEA comprises a 14 membered lactone fused to 1,3- dihydroxy benzene. It is a macrolide and also is a member of Beta-resorcinol which is known as a rare class of natural products. Molecular formula of the compound is C18 H22 O5. Result of previously executed experiment indicate that in the ZEAralenone structure there are ketonic, phenolic hydroxyls, olefinic and ester groups present.