Neurophysiological Background Of Addiction
Contents
Introduction
Addiction is a disease which may take many different forms. It is described as a state in which a person is unable to stop a stimulus seeking behaviour despite experiencing negative consequences. In simple terms impulsive behaviour becomes compulsive behaviour. This may be in reference to using pharmacologically active substances or engaging in a specific behaviour, such as gambling or exercising compulsively. When a person is addicted, the brain’s reward system starts to operate in a different way, making it dependent on the substance that the person is addicted to. After a period refraining from ingesting such a substance, a person may start to experience symptoms and demonstrating signs of withdrawal. Such abstinences, while unpleasant, are mostly not life threatening. However, in severe cases complications of withdrawal such as epileptic seizures or cardiac arrhythmias can lead to death (Camí and Farré, 2003).
The Brain And How It Communicates
The brain contains between 100 million and 100 billion neurons depending on the species. The different parts communicate through neurons mediated by small chemicals known as neurotransmitters. They are released from one neuron to the other through small gaps, known as synapses. These chemicals will then bind on the neighbouring neuron to convey the information further. All communication is dependent on the electrical charge of action potentials.
There are over 200 different neurotransmitters, and they can be categorized into two groups. Excitatory neurotransmitters, like glutamate and acetylcholine, which means they increase the likelihood of firing the action potential, and inhibitory neurotransmitters, such as serotonin and GABA, which decrease the likelihood. Dopamine is a neurotransmitter that acts both as an excitatory and inhibitory neurotransmitter and is connected with the reward system, making it an important player in addiction neurophysiology. To understand the reward system, we need to be familiar with certain structures of the brain and the tasks of the neurotransmitters.
Participating Structures Of The Central Nervous System
The brain is a complicated organ which consists of different parts working together to perform and coordinate specific functions. Certain areas in the brain are responsible for fundamental life processes like the regulation of respiration and heart rate while other areas are responsible for generating the pleasurable sensations associated with reward. What makes some drugs so dangerous in high doses is their association with the former, and what makes them so addictive is their association with the latter. These areas are (Hove et al, 2010) listed below, see figure 1 for visualization:
The Brainstem
The ventrolateral medulla and dorsolateral pons are sites important in the regulation of respiratory depth and rate as well as responsiveness to CO2 and hypoxia.
The Ventral Tegmental Area (VTA)
The VTA is located in the midbrain and is one of the main dopamine producing areas in the brain. The VTA has an especially important role in the reward system, by forwarding information to other areas of the brain.
The Amygdala
The amygdala is a collection of nuclei found in the temporal lobe and is considered a part of the limbic system, which is responsible for emotions. The amygdala causes us to feel fear and anxiety, but also controls the way we react to certain stimuli, like a threat or a dangerous situation. It also has a role in processing memory.
The Nucleus Accumbens (NAcc)
The NAcc is located in the basal part of the forebrain and is a major part of the ventral striatum, which is responsible for motivation and reward. It is connected to the VTA and therefore has an important role in the reward system as well. It is both associated with the limbic system and the motor system.
The Prefrontal Cortex
The prefrontal cortex is the most frontal part of the frontal lobe. This region is associated with executive functions, like planning, decision making, problem solving etc. It is also responsible for personality expression, behaviour and speech.
The Hippocampus
The hippocampus is located on the medial side of the temporal lobe and is also a part of the limbic system. Its main function is formation of memory and learning, but also spatial processing and navigation.
Figure 1: The Most Important Structures Related To Addiction, And Their Location In The Brain. |
The Functions Of Neurotransmitters
Dopamine
Dopamine is a catecholamine neurotransmitter that is released in the reward system, creating a feeling of euphoria and pleasure, the feeling driving addictions. It is synthesized from Tyrosine and the largest amounts of dopamine are found at the VTA and NAcc. There are at least 5 subtypes of dopamine and they act on different dopamine receptors. It is linked to many different functions such as movement, memory, sleep, learning, behaviour and attention. When there is a deficiency of dopamine, delayed and uncoordinated movements can be observed. If there is an excess, rapid, unnecessary movement occur such as repetitive tics (Baler et al, 2009).
Glutamate
Glutamate is an amino acid that functions as a neurotransmitter, which is synthesized within the brain. It causes excitatory action when it binds to its receptor. Glutamate is one of the most abundant receptors and transmitters, and is present in more than half of the synapses in the brain. The full extent of the different receptors glutamate act on is yet to be discovered but there are some receptors that have been identified for the glutamate. These are the 3 ionotropic receptors: NMDA, AMPA and kainate receptors. When they are activated, they will allow for sodium to flow into the postsynaptic neuron, depolarize it, making it more likely to fire. NMDA receptors are well suited to be involved in synaptic plasticity (changes) that occur in response to an experience, making it important in learning and memory (Danbolt and Zhou, 2014).
Gamma-Amino-Butyric-Acid (GABA)
GABA is an amino acid neurotransmitter and is synthesized from glutamate. In the mature brain it acts in an inhibitory matter. There are two types of GABA receptors. The GABAa is an ionotropic receptor and will, when activated, open for calcium to flow through and hyperpolarize the cell, making it less likely to fire. GABAb receptors, which are metabotropic (G-protein coupled) when they are activated, will open a potassium channel allowing potassium to flow out of the neuron, hyperpolarizing it. Because it is an inhibitory neurotransmitter, an increased amount of it will have a sedative effect. In simplified terms, alcohol and benzodiazepines share this mechanism of action (Sun and Wu, 2015).
Endorphins
Endorphins are endogenous opioid polypeptide compounds. They are produced by the pituitary gland and the hypothalamus in vertebrates during strenuous exercise, pain or stress. Endorphins acts on the opiate receptors which can be found all over the CNS, specifically in the brainstem, medial thalamus, spinal cord, limbic system and the hypothalamus, and are important receptors to perceive information about pain. There are 4 different types of endorphins named alpha, beta, gamma and sigma. The gamma opioid receptor will, when activated, inhibit the release of GABA which will strengthen the dopamine pathway within the reward system, which may lead to addictive behaviour (Leuenberger, 2006).
The Reward System
The reward system is the brain’s way of letting you know when you are doing something right. The action is, in evolutionary terms, perceived as beneficial to the organism. The reward system is based on the release of dopamine, therefore we often refer to it as the dopamine pathway. This pathway is influenced by natural behaviours such as ingesting food and having sex, but it can also be artificially influenced by addictive drugs or actions. These actions will stimulate neurons to release dopamine.
Commonly we divide the dopamine pathway into two main parts: the mesocortical and mesolimbic pathway. Together the two parts are referred to as the mesocorticolimbic pathway. They form the extended amygdala. The mesolimbic pathway connects the VTA with the nucleus accumbens. The VTA is also connected to the limbic system and prefrontal cortex. The essential parts of the limbic system are the amygdala and the hippocampus (Kumaresan and Pierce, 2006). The mesocortical pathway has branches from the VTA to many areas in the cortex. Dopamine release from the VTA is determined by the prefrontal cortex. It decides whether to initiate or stop an action, such as whether to sit or not when the owner tells the dog to do so. In the same way it may allow or prohibit the initiation of an addictive behaviour (Camí and Farré, 2003).
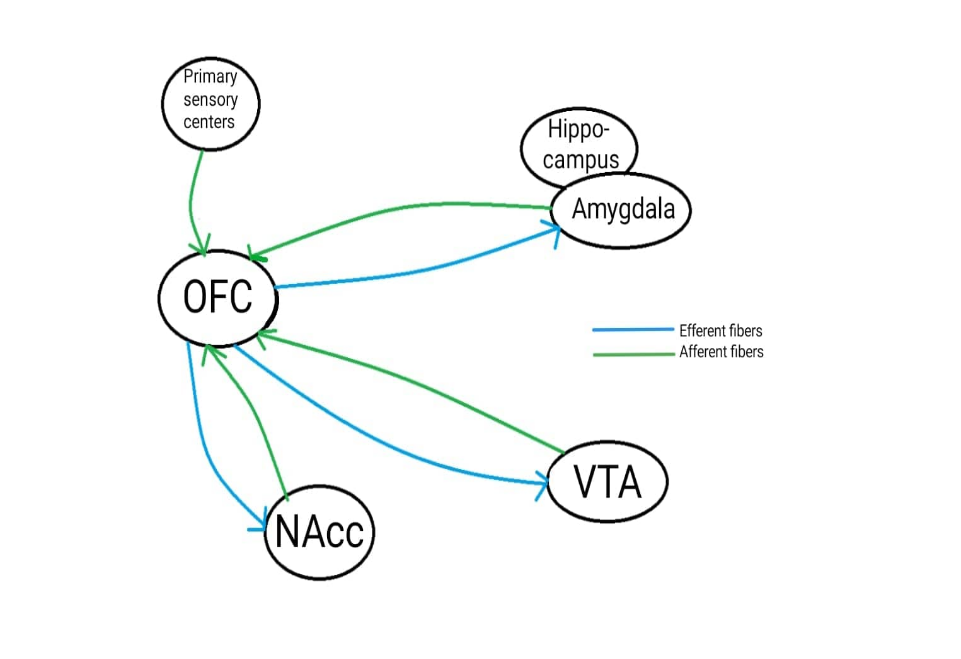
The frontal and orbital cortex together is named the orbitofrontal cortex (OFC) and is a part of the prefrontal cortex. The OFC is responsible for the conscious actions in the addiction, such as the need to take more (Camí and Farré, 2003). The OFC receives sensory input from the primary smell and taste centres, along with other brain centres of sensory organs. Fibers also travels bidirectionally from the amygdala. In addition, efferent fibres also travel to NAcc and VTA (see figure 2).
|
Figure 2: Overview Of The OFC. A simplified illustration showing the areas of the extended amygdala that will send and receive information to or from the OFC. |
The mesocorticolimbic pathway has projections of neurons from the NAcc to the VTA, this is what completes the full mesocorticolimbic pathway. The previously described connections between several areas in the brain have various effects, due to the different neurotransmitters used. We have the dopaminergic, GABAergic and the glutamatergic fibres (see figure 3). The dopaminergic fibers travel from the VTA to the amygdala, hippocampus and prefrontal cortex. When administering a drug that increases dopamine levels in the brain, positive or negative emotions and memory will be linked with the experience of the drug.The glutamatergic fibers travel from the OFC to the NAcc and VTA. The amygdala and hippocampus have glutamatergic fibers innervating the NAcc. The excitation of the VTA and NAcc will stimulate the production and release of dopamine, leading to a more intense feeling of reward. GABAercic fibers will travel from the NAcc to the VTA. This can lead to a slower dopamine release from the VTA, creating a sedative effect (Kumaresan and Pierce, 2006), (Chiara and Bassareo, 2006).
|
Figure 3: Pathway Of The Dopa-, Glutamate-, And GABA-ergic Neurons In The Mesocorticolimbic System. The figure gives an overview of the different connections in the mesocorticolimbic system, and their neurotransmitters. |
Drugs Abusing The Reward System, By Influencing The Dopamine Pathway
In general, addictive substances are addictive due to their ability to cause synaptic plasticity, this is modifications of the strength of synaptic transmission at pre-existing synapses. The NAcc integrates information from the cortical and limbic structures in order to mediate goal directed behaviours. Long lasting exposure to various classes of drugs may disrupt plasticity in this region and in this manner trigger drug seeking behaviour (Heinsbroek et al, 2016). By a one-time administration of an addictive drug, the strength of the synapses increases, but will after a short period of maximum 5 days return to its normal state. When there is increased and prolonged substance abuse, the plasticity remains and especially the VTA dopamine neurons becomes sensitized, requiring stronger stimuli to engage the reward system, see figure 4 (Kauer and Malenka, 2007).
Cocaine
When administering cocaine, dopamine reuptake by transporters is inhibited. The perception of a “high” correlates with the amount of cocaine present in the body (Volkow et al, 1997). When the amount of cocaine decrease, the dopamine levels are restored to a normal level. Senzitization of the dopamine receptors, leads to a higher concentration needed, and NAcc initiates the drug seeking behaviour to reach, a now higher, concentration of dopamine to give the previously felt "high".
Heroin
Heroin belongs to the pharmacological family of opiates. Opiates are direct receptor agonists that have similar effects as the body’s natural opioids; endorphins, enkephalins etc. The receptors are located all over the CNS including VTA and NAcc. Binding of opiates to these receptors leads to euphoria, analgesia, sedation and respiratory depression amongst other effects. The opioids will either directly inhibit GABAergic accumbal output neurons (Hakan and Henriksen, 1989) or will inhibit the release of neurotransmitters from glutamatergic neuron terminals in NAcc (Jiang and North, 1992). If GABA release is inhibited in the VTA, it will disinhibit dopaminergic neurons, meaning there is an increased dopamine release in NAcc.
Cannabis
Cannabis sativas main active ingredient is delta-9-tetrahydrocannabinol (THC). It works by influencing the endocannabinoid system. The body has similar natural transmitters referred to as endocannabinoids (eCB) (Zou and Kumar 2018). Strong influx of Ca2+ to glutamatergic and GABA releasing synapses will trigger the synthesis of eCB. These lipophilic molecules travel along the presynaptic cleft to the postsynaptic and binds to eCB receptor 1. This binding depresses the release of neurotransmitters of the specific terminal. If there is a prolonged exposure of eCB, it will cause long-term depression, also known as activity-dependent weakening of synaptic transmission. This is affecting the reward system through allowing for the release of dopamine (Kauer and Malenka, 2007).
|
Figure 4: Drugs Of Abuse And Their Effect On Plasticity In VTA. Drugs and stress (as indicated in the box) results in potentiation of the synapses and their effect. One possible way this is happening is the increase of AMPAR postsynaptic receptors at glutamatergic synapses. This increase in synapses is reversible by metabotropic glutamate receptor (mGluR). When glutamate binds to the NMDAR, nitric oxidase synthase (NOS) is activated, this leads to the production of nitric oxide (NO). NO is a diffusible messenger that is released from postsynaptic neurons and activates guanylate cyclase (GC) in neighbouring presynaptic inhibitory terminals. When there is an increase of cGMP, GABA will be released to bind to GABAa receptors. Morphine can prevent the production of cGMP by inhibiting the signals of NO.
Treatment Of The Addicted Brain
For the brain to get “back to normal”, the brain must go through another period with adaptation and change, only this time adapt back to its own chemical signals. This may be a time-consuming process, see figure 5. The human body and brain have a great ability to adapt and change in response to environmental changes. If there are any damaged areas in the brain, the brain is capable of working around these areas and building up new pathways. This allows the brain to still maintain function by sending messages along a different route.
As for treatment, there are multiple treatments but no cures for addiction. Addiction is thought of as a chronic disease, and as with other long-lasting diseases, the most important key is to learn to manage and control the disease. Some pharmacological strategies can be employed in certain situations. Buprenorphine and methadone have shown great success in the treatment of opioid addiction. The function of these medications is to decrease the drug’s reward value, in other words, make the drug experience less exciting or even unpleasant. The medications affect the same neurotransmitter system as the system of the drug being abused. Bromocriptine and apomorphine function to increase the release of dopamine, and to increase the drug value of the natural reinforcement (Fowler et al, 2004).
A common experience of individuals trying to get clean is the so-called relapse. A relapse is a return to drug abuse after a period of intended cessation. A relapse back to drug-abuse can be dangerous, depending on the type of drug and the duration of abstinence. After a period without drugs, the brain becomes less sensitized to the drug exposure. This is not always something an addict considers, as he/she is accustomed to the drug dosage that was used before quitting. In the case of opioid addiction overdoses are very common after a relapse due to the induction of respiratory depression (Camí and Farré, 2003).
|
Figure 5: The figure illustrates the brain’s ability to recover from drug abuse. The first brain scan show a normal brain’s activity, without any drug abuse. The second brain scan show a brain after 1 month without methamphetamine. The third brain scan shows a brain after 14 months without methamphetamine, on its way to full recovery. |
Discussion
Research aimed at increasing our understanding of the neurophysiology of addiction is imperative if we are to develop effective treatments for addiction. Decades of public policy aimed at reducing the availability of illicit substances and treating the consequences have largely failed from both a medical, sociological and legal point of view. The most promising results in addiction medicine, are reported from pharmacological interventions. There is a trend towards viewing addiction more in terms of a disease rather than primarily a moral or legal shortcoming. As such, our strategies in dealing with it should be guided by the evidence emerging from both experimental and clinical studies. Animal models can be very useful in trying to better understand the underlying physiology as well as the mechanisms at work when treating addiction. Such models can allow us to directly study pharmacological interventions on different pathways. There are in existence well established and validated small animal addiction models. These are cost effective and widely regarded as an ethically defensible way to find candidate treatments suitable for clinical trials. Due to increasing global public health concerns surrounding addiction, in particular to opioids, this is an extremely promising field of study both with regards to the ability to secure funding and its potential impact on public health outcomes (Kumaresan and Pierce, 2006).
Conclusion
In the neurophysiology of addiction the reward system generally and dopamine specifically are the most important components when turning a healthy brain, into a craving brain. If the organism experiences a pleasurable sensation, the amygdala will relate emotions to the sensation and the hippocampus will process and help store the memory of this feeling, the circumstances and the surroundings so that it can repeat the experience again. The NAcc will integrate cortical and limbic inputs and participate in the motor functions of the action, for example, administering more drugs (Heinsbroek et al, 2016). The prefrontal cortex will help in focusing on the experience, as well as the conscious decisions of deciding whether or not to continue. The basis of the function of the reward system is dopamine, as it drives the organism to perform good and productive tasks as it reinforces the brain with a highly pleasurable sensation. The abuse of harmful drugs alters the dopamine levels in the brain, this causes plasticity and the level of dopamine required for the brain to maintain normal function increases. The need for higher dopamine levels is part of what we call addiction. The craving brain has the physiological potential to be able to return to a healthy brain, if it’s given enough time. The difficulties involved in getting an individual to go along with such a period of drug cessation in order to allow the brain to return to its physiological baseline, is the basis for the challenges in addiction medicine.
References
Alexander, G. C., Clark, T. W., Courtwright, D. T., Eadie J. L., Hwang C. S., Kolodny, A., Kreiner, P. (2015): The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annual review of public health 36: 559-574.
Baler, R., Fowler, J. S., Telang, F., Volkow, N. D., Wang, G. J. (2009): Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56 (Suppl 1): 3 - 8.
Bassareo, V., Chiara, G. D. (2007): Reward system and addiction: what dopamine does and doesn't do. Current Opinion in Pharmacology 7 (1): 69 - 76.
Camí, J., Farré, M. (2003): Mechanism of Disease: Drug Addiction. The New England Journal of Medicine: 975 - 986.
Chen, A. D., Dewey, S. L., Fowler, J. S., Gatley, S. J., Hitzemann, R., Logan, J., Pappas, N., Volkow, N. D., Wang, G. J. (1997): Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386, 830–833.
Danbolt, N. C., Zhou, Y. (2014): Glutamate as a Neurotransmitter in the Healthy Brain. Journal of Neural Transmission 121 (8): 799–817.
Fowler, J. S., Volkow, N. D., Wang, G. (2004): The Addicted Human Brain Viewed in the Light of Imaging Studies: Brain Circuits and Treatment Strategies. Neuropharmacology 47 (January): 3–13.
Hakan, R. L., Henriksen, S. J. (1989): Opiate influences on nucleus accumbens neuronal electrophysiology: dopamine and non-dopamine mechanisms. J. Neurosci. 9, 3538 - 3546.
Heidbreder C. (2011): Advances in animal models of drug addiction. Molecular and functional models in neuropsychiatry pp 213-250.
Heinsbroek, J. A., Gipson,C. D., Kalivas, P. W., Kupchik, Y. M., Roberts-Wolfe, D., Scofield M. D., Smith, A. C., Spencer, S. (2016): The Nucleus Accumbens: Mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacological Reviews 68 (3): 816-871.
Herman, M. A., Roberto, M. (2015): The Addicted Brain: Understanding the Neurophysiological Mechanisms of Addictive Disorders. Frontiers in Integrative Neuroscience 9 (March).
Hove, K., Sand, O., Sjaastad, O. V. (2010): Physiology of Domestic Animals 3: 111 - 176.
Jian, Z. G., North, R. A. (1992): Pre-and postsynaptic inhibition by opioids in rat striatum. J. Neurosci. 12, 356-361.
Kauer, J. A., Robert C. M. (2007): Synaptic Plasticity and Addiction. Nature Reviews Neuroscience 8 (11): 844–858.
Koob, G. F. (1992): Drugs of Abuse: Anatomy, Pharmacology and Function of Reward Pathways. Trends in Pharmacological Sciences 13 (5): 177–84.
Kumaresan, V., Pierce, R. C. (2006): The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and Biobehavioral Reviews 30 (2): 215 - 238.
Leuenberger, A. (2006): Endorphins, Exercicse, and Addictions: A Review of Exercise Dependence. The Premier Journal for Undergraduate Publications in the Neurosciences: 1 - 9.
Sellers, E. M., Tomkins, D. M. (2001): Addiction and the Brain: The Role of Neurotransmitters in the Cause and Treatment of Drug Dependence. CMAJ: Canadian Medical Association Journal 164 (6): 817–21.
Sun, D., Wu, C. (2015): GABA Receptors in Brain Development, Function, and Injury. Metabolic Brain Disease 30 (2): 367–379.
Wise, R. A. (2004): Dopamine, learning and motivation. Nature Reviews Neuroscience 5: 483 - 494.
Wise, R. A. (2006): Role of Brain Dopamine in Food Reward and Reinforcement. Philosophical Transactions of the Royal Society B: Biological Sciences 361 (1471): 1149–58.
Zou, S., Kumar, U. (2018): Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int J Mol Sci 19(3): 833.
Additional Material
Khan Academy. Reward pathway in the brain. Retrieved April, 2019 from https://www.khanacademy.org/science/health-and-medicine/mental-health/drug-abuse-and-drug-addictions/v/reward-pathway-in-the-brain
UVMB, Department of Physiology and Biochemistry. Physiology of the Nervous System [Powerpoint presentation]. Retrieved April, 2019.
Figure refrences
Figur 1 - Open google search, free to use: Wikipedia. Dopamine pathways. Retrived April 2019 from https://he.m.wikipedia.org/wiki/%D7%A7%D7%95%D7%91%D7%A5:Dopamine_pathways.svg
Figur 2 - Self drawn, inspired by: Camí, J., Farré, M. (2003): Mechanism of Disease: Drug Addiction. The New England Journal of Medicine: 975 - 986.
Figur 3 - Self drawn, inspired by: Camí, J., Farré, M. (2003): Mechanism of Disease: Drug Addiction. The New England Journal of Medicine: 975 - 986.
Figur 4 - Self drawn, inspired by: Kauer, J. A., Robert C. M. (2007): Synaptic Plasticity and Addiction. Nature Reviews Neuroscience 8 (11): 844–858.
Figur 5 - Open google search, free to use: Flickr. Addiction-brain recovery. Retrieved April 2019 from https://www.flickr.com/photos/nida-nih/8019773180