The Effect of Ageing on Cardiovascular Function
Contents
Introduction
In this essay, the correlation between ageing and the function of the cardiovascular system is discussed. The cardiovascular system, as the transport system of the body, is responsible for variety of vital functions. The cardiovascular system consists of the heart and the blood vessels which are branching from it. Through it, oxygen can be transported to all body tissues, as well as other nutrients and hormones, and cellular waste products can be transported to their final elimination. Ageing processes have been proved to have variety of effects on this system, mainly due to histological changes in the cardiovascular tissues, which leads to certain dysfunctions. A wide variety of factors exert changes on this system. In this essay the major effect will be discussed. The findings represented in this essay, refer to a healthy population who do not suffer from any background cardiac disease.
Correlation between ageing and cardiovascular function
Age-related changes in the heart rate
Based on variety of previous studies, the maximal exercise heart rate declines with age, however, the average daily heart rate is not affected by ageing. (Kostis et al, 1982) Heart rate can be defined as the number of cardiac contractions per minute.
Studies have suggested that this decline in heart rate among elderly individuals, might be due to a decline in the capacity of the sinus node to increase the heart rate. The sinus node is the natural pacemaker of the heart. It is located at the upper part of the wall of the right atrium, and responsible for the continuous generation of excitation, which leads to the cardiac contractions. As a result of ageing, the sinus node does not transmit the electrical impulses at the same efficiency, and the heart contracts less, leading to a lower heart rate.
In addition, delayed recovery of heart rate after exercise has been observed. It is suggested to be because the elderly population use their reserve more than the young population, as they are more dependent on the anerobic metabolism to produce the energy needed. Such process can be explained by the redistribution of the circulatory system of the body. The cardiovascular regulation may rearrange the blood distribution according to the need of the organism. When the individual is under vigorous physical exercise, more blood will have to be distributed to the muscles. To provide this, the cardiac output will increase, thus, heart rate will increase as well.
Moreover, the sympathetic activity has also a major effect on the heart rate in response to exercise. Norepinephrine levels in the plasma of individuals were measured during the day and the night, at rest, and in response to postural changes or static positions. Norepinephrine, as a neurotransmitter with sympathetic effect, has chronotropic effect on the heart. Thus, by stimulation of β1 receptors, it leads to an increased heart rate. Study (Kostis et al, 1982) showed that Norepinephrine levels were higher in the elderly population, which resulted in greater chronotropic effect and increased heart rate. Those findings explained the delayed recovery of heart rate in response to exercise, which was highly observed with ageing.
Age-related changes in the heart valves
The heart contains four valves, which allow the passage of blood from one compartment to another, only in one-direction. It prevents the backflow of blood in the opposite direction. They can be either subdivided into two atrioventricular valves (tricuspid valve and bicuspid valve) and two semilunar valves (pulmonary valve and aortic valve). The tricuspid valve is located between the right atrium and the right ventricle, the pulmonary valve is located between the right ventricle and the pulmonary artery, the bicuspid (mitral) valve is located between the left atrium and the left ventricle, and the aortic valve is located between the left ventricle and the aorta. Each of those valves is composed of three cusps (except of bicuspid valve which contains two), papillary muscles and tendinous chords in between them.
The anatomy of the cardiac cusp (Figure1) is necessary for understanding the changes followed by ageing. Each valve is composed of a few flaps called cusps. The cusps are named based on their position. The pulmonary valve contains left, right and anterior cusps. The aortic valve contains left, right and posterior cusps. The tricuspid valve contains anterior, posterior and septal cusps, and the bicuspid valve contains anterior and posterior cusps.
At the edge of each cusp there is a nodule which keeps it tighter, and a free edge.
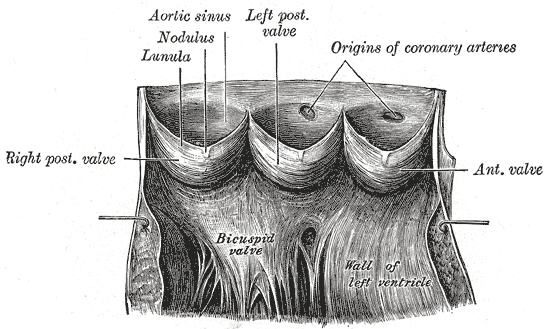
Figure 1 Anatomical structure of the cardiac valve |
As a result of ageing, a few structural changes of these valves have been observed. (Pomerance, 1967) First changes will be described regarding the bicuspid (mitral) valve. Followed by ageing, the anterior cusps experiences thickening of its nodule. Pathological conditions as senile sclerosis and atheromatosis (lipid deposition) are related to it. As a result, lesions appeared near the insertions of the tendinous chords. In more severe cases, it could be seen even between the chords. Regarding the posterior cusp of the mitral valve, several changes have been observed. A strong correlation was found between ageing and calcification of this cusp, mainly in females. Calcification describes the formation of calcium deposits in cusp, resulting in narrowing of the opening of the valve – valve stenosis. In addition, another structural change was observed firstly in dogs and named as “Billowing sail distortion”. In humans it is named “Mucoid degeneration”. This condition occurs when the center of the cusp thickens and becomes opaque and voluminous. As a result, the tendinous chords become extremely weak and may even rupture. Other structural age-related changes appeared in the aortic valve as well. Similar to the bicuspid valve, a thickening of the modules was observed. Moreover, adhesions and broadening of the commissures of the valves increased due to ageing. Regarding the tricuspid valve, the main change which is age-related is similar to the thickening of the nodule in the bicuspid and aortic valves. However, a special finding was found here. A local ulceration appeared in the superficial part of the plaque of the anterior cusp. Unlike the other valves, the pulmonary valve only slightly changes with age. Only thickening at the centers of the free edges appeared.
The reason for these changes is found to be hemodynamic factors. The flow of blood through the bicuspid and aortic valves becomes less forceful with age, and it stimulates a hemodynamic response, for instance, it is responsible for hyperplasia (increased amount of organic tissue that results from cell proliferation). This hyperplasia leads to the diffuse opacity which is observed in the bicuspid valve, and the commissural adhesions which are observed in the aortic valve.
Age-related changes in the myocardial tissue
The myocardium is the muscular tissue of the heart. It is composed of two types of components: the contractile component and the non-contractile components. The contractile components refer to excitable cardiomyocytes as the pacemaker cells, the conductive system (His bundle, Tawara bundle and Purkinje fibers) and the working fibers. The non-contractile components refer to the ECM (extracellular matrix), containing mainly collagen, and other elastic elements and proteins.
Ageing processes of the heart are characterized by myocardial remodeling, including decreased cardiomyocyte number and increase in their size, and increased ECM. (Kwak, 2013) Through the remodeling of ECM due to ageing, there is a massive loss of the contractile components, mainly in the left side of the heart. A common tissue response to ECM remodeling, which is associated with ageing, is fibrosis. Fibrosis describes the biological process by which the contractile components are replaced by fibrous tissue. Among variety of mechanisms, it can occur due to activation of cardiac fibroblasts. These fibroblasts synthesise high concentrations of collagen, leading to collagen deposits and formation of a fibrotic scar (Figure 2) (Hinderer et Schenke-Layland, 2019). As scars act as electrical insulators, cardiac conductivity becomes interrupted, leading to impairment of the cardiac contractions which can result in diastolic and systolic dysfunction and even heart failure.
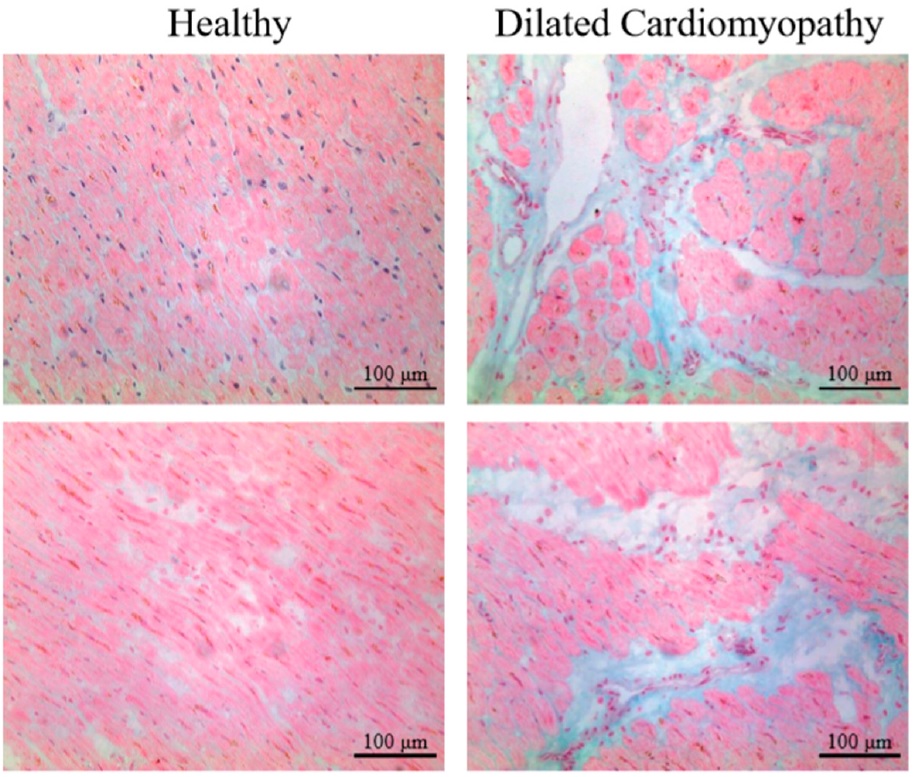
Figure 2 Healthy and affected myocardium |
Age-related changes in the cardiac autonomous nervous system
The cardiac autonomous nervous system (ANS) exhibit both extrinsic and intrinsic regulation mechanisms. The extrinsic regulation has sympathetic and parasympathetic effects, while the intrinsic regulation is based on myogenic tone regulation, endothel related regulation and metabolites related regulation.
The cardio-vasomotor centers are located in the medulla oblongata. Three main areas can be distinguished there, based on their function: the pressor area, the depressor area and the cardioaccelerator area. These areas are a diffused network of neurons, which are responsible for the regulation of the cardiovascular system. The pressor area has a spontaneous sympathetic activity. It has positive chronotrop and dromotrope effects on the heart, meaning that it increases the frequency, contraction time and speed of conduction, respectively. Consequently, cardiac output increases, peripheral resistance (TPR) and so the blood pressure increases as well. Unlike the pressor area, the depressor area does not have spontaneous activity. When stimulated, it uses the vagus nerve to apply negative chronotrop and dromotrope effect of the heart. Furthermore, it can directly inhibit the activity of the pressor area by its inhibitory interneurons, achieving vasodilation (by decreasing the sympathetic vasoconstrictor tone). As a result, blood pressure will decrease. Under resting conditions, the depressor area continuously inhibit the pressor area, so the vagus nerve determines the work of the heart.
Cardiomyocytes (mainly the round cells of the SA node) contain β1 adrenergic receptors. When activated, it stimulates the G-protein to increase the activity of adenylate cyclase, thus, to increase the IC (intracellular) cAMP levels. As a result, MDP (maximal diastolic potential) shifts upward and SDD (spontaneous diastolic depolarization) increases, thus, the threshold is reduced. Consequently, the heart rate increases. Stimulation can be by sympathetic innervation or by the neurotransmitters epinephrine and norepinephrine. Simultaneously, parasympathetic effect (acetylcholine release) is inhibited by the neurotransmitter NPY, in order to enhance the sympathetic effect. In order to decrease the heart rate, parasympathetic effect is favored. It occurs by the release of acetylcholine which activate mACh receptors on the round cells of the SA node, resulting in the opposite mechanism.
Chadda et al (2018) showed that ageing processes are related with the decrease of β adrenergic receptors response and density. This occurs due to changes in the G-protein and adenyl cyclase which lead to their dysfunction. This changes lead to decreased IC cAMP levels, thus to a decreased response of β adrenergic receptors to stimulation. It can explain the decline in heart rate with ageing, which was discussed earlier.
Age-related changes in vascular morphology
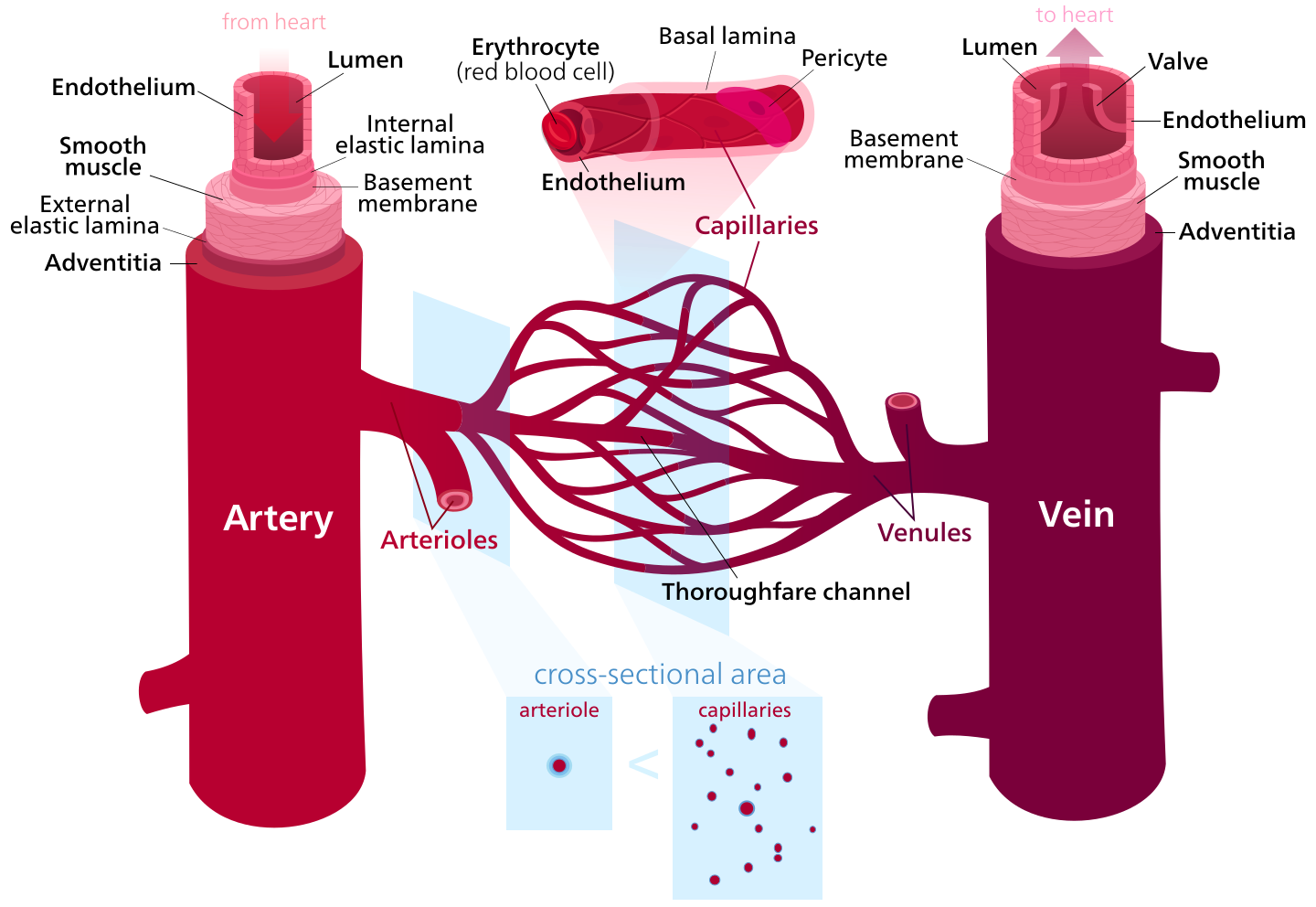
Before describing the changes in the vascular system in relation to ageing, the structure of blood vessels will be discussed. The morphology difference between tissues of different blood vessel types has a critical physiological importance. (Figure 3)
Arteries are elastic blood vessels which take blood away from the heart to the body tissues. Their elasticity allows them to carry out this function. The outer layer of an artery is called tunica externa and is composed of collagen fibers and elastic fibers. The next layer is the tunica media, which is composed of wide smooth muscle, elastic fibers, and collagen fibers. The innermost layer is the tunica intima which is in direct contact with the blood flow, and it is composed of epithelial cells. Arteries can be further categorized into elastic arteries, muscular arteries, and arterioles, depending on the ratio between the components in their wall. For instance, elastic arteries as the aorta, are rich with elastic elements, while muscular arteries exhibit more smooth muscle.
Veins are blood vessels which carry blood from body tissues into the heart. The walls of veins are composed of main three layers. The outermost layer is the tunica externa which is composed of connective tissue. The middle layer is the tunica media which is built of smooth muscle, and the tunica intima, which is innermost layer, is made of endothelial cells. A special structure for the veins is the valves, which aim to prevent the backflow of blood. Unlike the arteries, veins are not elastic.
Capillaries are the smallest blood vessels in the body, as they are built on one endometrial layer only. They can be subdivided into three types: continuous capillary, fenestrated capillaries, and sinusoidal capillaries. Continuous capillaries contain uninterrupted endothelium, fenestrated capillaries have pores within it, and sinusoidal capillaries are irregular spaces filled with blood, resulting from wider pores in the endothelium.
|
Figure 3 Histological structure of blood vessels |
As a result of ageing, structural changes occur in the vascular walls of elastic arteries, which lead to important physiological consequences. Among those changes, the loss of elastin in the arteries has a major importance. Studies showed that with older age, elastin content in the tunica media decreases and progressively replaced by collagen. The existing elastin elongates and loses its elastic recoil properties. It leads to an abnormal increased diameter of the arteries and results in a thickening of the tunica intima mainly, but also the intima. The highly increased collagen content limits the vascular distensibility of the arteries, meaning that their ability to take up more blood when blood pressure increases is reduced. The reduced distensibility leads to decreased compliance as well, and to increased pulse pressure, which is highly observed in the elderly population. This thickening of the tunica intima and the tunica media was found to be associated with variety of cardiovascular diseases as atherosclerosis, myocardial infarction, and stroke. (Jakovljevic, 2018), (Thijssen, 2015), (Steppan et al, 2011).
Moreover, there is a strong correlation between ageing and impairment endothelium regulation. Normally, endothelial cells in the tunica intima of arteries secrete certain humoral factors which are responsible for important physiological processes. Those factors can be categorized into two group: EDRF (endothelium-derived relaxing factor) and EDCF (endothelium-derived contracting factors). Among the EDRF, there are NO (nitrogen monoxide) and Prostacyclin hormone. Those are responsible for smooth muscles relaxation of the arteries. When NO secretion from endothelium cells increases, it activates the guanyl-cyclase system, resulting in increased cGMP levels. cGMP causes smooth muscle relaxation, leading to vasodilation of the arteries. Prostacyclin increases NO levels, but also has a direct effect on smooth muscle relaxation, by increasing cAMP levels. Vasodilation is important for increased perfusion when needed. Followed by this, the blood flow is higher, resulting in decreased blood pressure. Among the EDCF, there are endothelins and angiotensins. Those are vasoconstriction factors which are responsible for the contraction of arteries. By this, blood flow is slower, thus, blood pressure is higher.
Due to ageing, endothelial cells appeared to be abnormally flattened and enlarged, containing polyploid nuclei. These structural abnormalities lead to impaired activity. The levels of NO and Prostacyclin decrease and the levels of angiotensins and endothelins increase in the elderly population. Thus, vasoconstriction is more favored than vasodilation, leading to higher blood pressure with ageing. (Jakovljevic, 2018)
Cardiomyopathy of ageing wild and captive non-human Great Apes
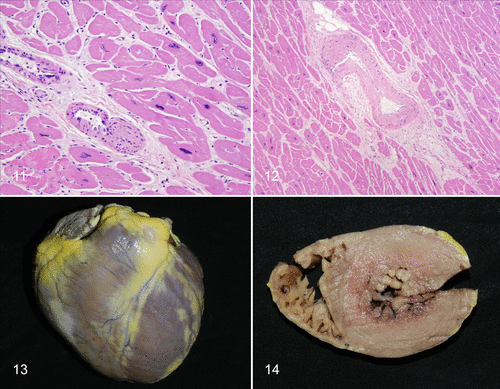
According to recent studies (Baldessari et al, 2013), (Lowenstine et al, 2016) cardiovascular diseases are described as the most common cause of sudden death in aged captive great apes (mainly in chimpanzees, gorillas and orangutangs). Among all cardiac diseases, fibrosing cardiomyopathy is the most common disease to be observed, mainly in captive gorillas. Fibrosing cardiomyopathy in ageing great apes occurs due to the remodeling of ECM of the cardiomyocytes, leading to replacement of healthy cardiomyocytes by excessive collagen deposit, forming a scar tissue (fibrosis). Thus, cardiac conduction and contractions are interrupted, and heart failure can occur, as explained earlier.
Unlike in humans, the exact mechanism for developing fibrosing cardiomyopathy in non-humans great apes is still unknown and under intensive research. In humans, associated vascular diseases or inflammatory processes usually accompany this condition, however, fibrosing cardiomyopathy appears to develop in great apes spontaneously without background diseases, only by the intense synthesis of collagen by fibroblasts (Hinderer et Schenke-Layland, 2019). Yet, a recent study (Lowenstine et al, 2016) suggests that left ventricular hypertrophy (enlargement) is a possibility to be an underlying cause for fibrosing cardiomyopathy. (Figure 4)
|
Figure 4 Heart from a geriatric female chimpanzee with fibrosing cardiomyopathy and left ventricular hypertrophy (Lowenstine et al, 2016) |
Regarding to wild great apes, lower prevalence of mortality was observed, but much wider research should be done. (Lowenstine et al, 2016)
References
Other sources
1. Figure 1: https://en.wikipedia.org/wiki/Aortic_sinus#/media/File:Gray497.png
2. Figure 2: https://commons.wikimedia.org/wiki/File:Blood_vessels-en.svg