Celiac Disease: Incidence and Causes
Celiac Disease, hereby CD, is a disease that is triggered by the ingestion of gluten (Guandalini & Assiri, 2014). It results in a disorder in the small intestine due to an inflammatory reaction to the gluten molecule (Guandalini & Assiri, 2014). Approximately 1 % of the European and USA populations suffer from CD (Guandalini & Assiri, 2014). The name celiac derives from the Greek "koiliakos", meaning "belly" (Woodward, 2010). Only 10% to 15% of this affected population have been diagnosed and treated (Guandalini & Assiri, 2014). There is a wide array of clinical signs for CD, many of them are not directly related to the gastrointestinal tract and this may therefore delay the diagnosis (Kagnoff, 2012). This is a disease that can affect people of any age and the treatment is a lifelong gluten-free diet.
Contents
Signs and symptoms
The primary reaction is the inflammation and damage of the small intestine and as a result the absorbance of important nutrients such as iron, folate, vitamin B12, calcium, proteins, fats and fat-soluble vitamins suffers. This causes secondary reactions such as anemia, osteoporosis and abnormal bleeding. CD is frequently found together with other autoimmune diseases, for example type 1 diabetes mellitus, autoimmune thyroiditis, autoimmune hepatitis, dermatitis herpetiformis and autoimmune alopecia (Schuppan, et al., 2009). Physicians should be aware for atypical cases, so as to improve diagnosis and avoid complications (Ciclitira, et al., 2005; Mouterde, et al., 2013). Symptoms mainly manifest themselves in the gastrointestinal tract, but there are many extra-intestinal symptoms too.
Gastrointestinal tract
Abdominal pain and diarrhea are the most common symptoms. Rarer symptoms are electrolyte disturbance, hypotension and lethargy, these symptoms are particular when there is a late diagnosis (Guandalini & Assiri, 2014). Younger patients (children under 2 years) usually show more symptoms such as diarrhea or malabsortive manifestations, which are considered to be the more "classical" symptoms (Reilly & Green, 2012). Older children and adults are more likely to show symptoms that are not directly connected to the gastrointestinal symptoms (Reilly & Green, 2012).
Patients that have symptomatic CD for a long time without treating it are at an higher risk of developing cancer as enteropathy-associated T-cell lymphoma (EATL) and small bowel adenocarcinoma of the gastrointestinal tract (Schuppan, et al., 2009). Refractory CD can develop in 5-10% of adults with undetected and untreated celiac disease for a long period. It occurs when your intestine fail to heal. These patients do not respond to or experience a relapse even though they are on a strictly gluten-free diet (Schuppan, et al., 2009). Approximately 50% of patients with refractory CD develop EATL within five years of diagnosis (Schuppan, et al., 2009).
Extra-intestinal manifestations
CD presents with a wide variety of symptoms such as tiredness, nausea, reduced appetite, stomach pains, vomiting, abdominal gas, bloating, hard or loose stools and weight loss (Rosén, et al., 2014). CD can affect the growth of children in a negative way, and may also delay puberty (Newton & Singer, 2012). The disease may also affect the bone mineral content and cause osteoporosis, which increase the risk of bone fractures (Kurppa, et al., 2014). Other symptoms to CD can be reproductive disorder, splenic hypofunction, dental enamel defects, cardiac problems and liver damage (Kurppa, et al., 2014).
Symptoms such as skin disease have also been described in CD patients, and increased rates of psoaris have been observed (Abernavoli, et al., 2006; Samasca, et al., 2014). An associated condition is Dermatitis Herpertiformis, which causes itchy blistering skin, mostly effecting knees, elbows, back, buttocks and occasionally also within the mouth (Ciclitira, et al., 2005). Although it is rare for patients with CD have dermatitis herpertiformis, all patients with dermatitis herpertiformis have some degree of enteropathy (Woodward, 2010).
What causes Celiac Disease?
Gluten, a protein found in barley, wheat and rye, can cause CD. In some people who are exposed to it, an enzyme called tissue transglutaminase (tTG) alters the gluten in such a way that it causes an immune response, which leads to inflammation of the gut. Small projections known as villi line the gut and are important in increasing the surface area available for nutrient absorption. In CD, these villi are flattened and destroyed, preventing the normal absorption of nutrients.
Pathogenesis
CD occurs as a result of environment influences in genetically predisposed individuals (Guandalini & Assiri, 2014). According to Schuppan (2009) CD can be characterized by (1) a defined trigger, such as gluten proteins found in wheat and other cereals, (2) The presence of human leucocyte antigen (HLA) class II haplotypes DQ2 or DQ8, and (3) the production of autoantibodies to the enzyme tissue transglutaminase (tTG).
(1) CD is triggered by proteins in cereal grains such as wheat, barley and rye. Gluten is the term used to describe the disease-activating proteins in these cereals. Gluten comprises of two major protein types, gliadins and glutenins, both of which possess disease-activating peptides rendering them resistant to complete digestion (Kagnoff, 2007).
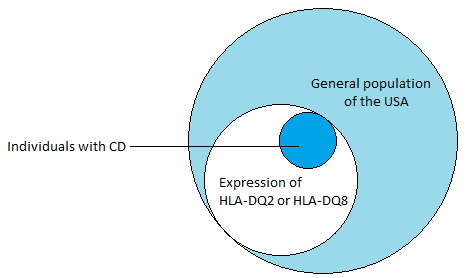
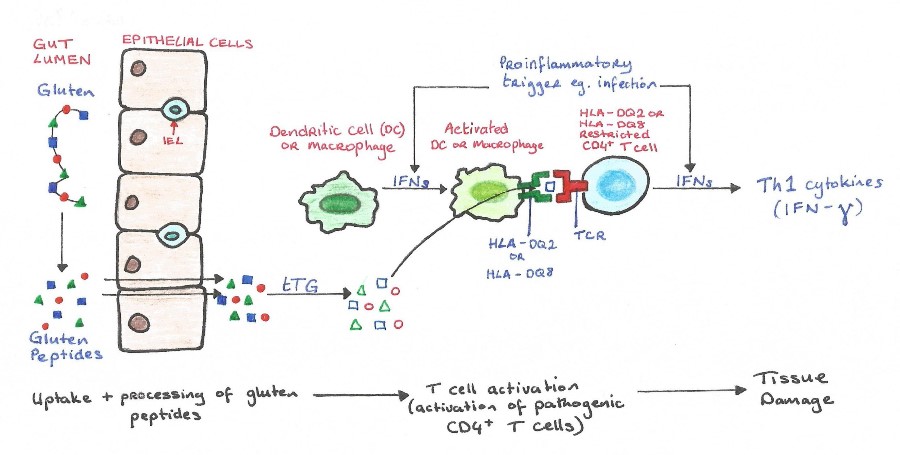
(2) The presence of MHC class II HLA-DQA alleles, HLA-DQ2 and HLA-DQ8, is the most important genetic susceptibility factor contributing about 40% (see Figure 1) to the risk of developing CD (Schuppan, et al., 2009). These molecules are expressed on by antigen presenting cells in the lamina propria of the small intestine, and are responsible for presenting gliadins (gluten proteins) to CD4+ T cells, which triggers an inflammatory immunological response (Guandalini & Assiri, 2014). tTG has a high avidity for the gluten peptides and is crucial in the deamination of gluten epitopes (Ciclitira, et al., 2005). tTG works by causing the deamination of glutamine residues found in gluten peptides (Kagnoff, 2012). Once the deaminated peptide is bound to the HLA-DQ2 or HLA-DQ8, the complex can activate the CD4+ T cells resulting in the inflammation through the secretion of IFN-ɣ (see Figure 3). IFN-ɣ is thought to play a key role in the downstream regulation of mucosal damage as it has been shown the neutralization of IFN-ɣ prevents mucosal damage (Kagnoff, 2007).
(3) The final characteristic of CD is the presence of autoanitbodies which are generated as part of the immunological response to tTG. These autoantibodies (such as anti-tTG IgA and antiendomysium IgA) are highly specific to the condition and can be used for validation and diagnostic purposes.
|
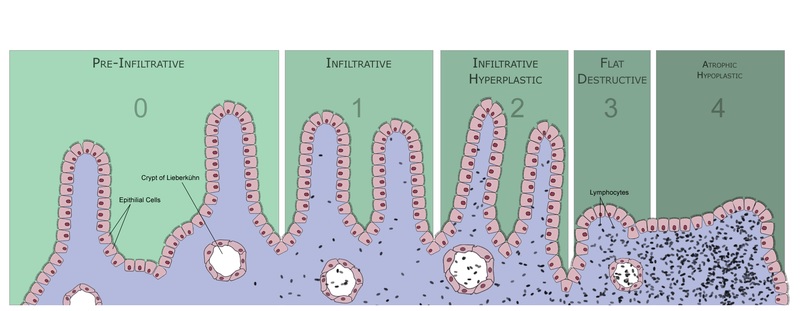
The inflammation of the small intestine causes flattening of intestinal villi, infiltration of lymphocytes into the epithelium and an increase in the depth and density of intestinal crypts. Marsh introduced a scheme to grade the intensity of intestinal damage (see Figure 2):
Type 0: Pre-infiltrative stage – normal.
Type 1: Infiltrative stage – increase in intraepithelial lymphocytes (IELs).
Type 2: Hyperplastic stage – development of hyperplastic crypts, increased IELs.
Type 3: Destructive stage – villous atrophy to various degrees.
Type 4: Hypoplastic stage – occurs in those unresponsive to gluten withdrawal. May develop malignant complications.
|
Refractory CD is where patients with CD experience a relapse of symptoms and intestinal damage despite being on a gluten free diet (Woodward, 2010). Refractory CD can develop in 5-10% of adults with CD, which often have gone undiagnosed for a long period of time. It can be divided into two types. Refractory CD type 1 has a normal IEL phenotype, is very responsive to immunosuppressants such as corticosteroids, and doesn’t typically evolve into enteropathy-associated T-cell lymphoma (EATL). Refractory CD type 2 has an abnormal IEL phenotype and is considered a pre-malignant condition. Approximately 50% of patients with this type develop EATL within five years of diagnosis (Schuppan, et al., 2009). The treatment of EATL is based on cytotoxic agents, similar to that of other lymphomas, but despite aggressive therapies the prognosis is poor. The survival rate of patients with EATL is only 15-20% in two years (Al-Toma, et al., 2007).
|
Diagnosis
Serological markers can be used to diagnose CD. The two main autoantibodies, which can be used as serological markers, are anti-tTG IgA and antiendomysium IgA, and these can be used to identify the presence of type 1 or type 2 CD. Anti-tTG IgA is preferred for test screening, while antiendomysium IgA has the highest specificity. Recently, a third autoantibody has been identified which is produced against deamidated gliadin peptides (DGPs) and the presence of DGP-IgG and DGP-IgA can be used to identify active CD. DGP-IgG is considered more sensitive and specific than DGP-IgA (Guandalini & Assiri, 2014).
If CD is suspected due to raised serological autoantibodies, the diagnosis is confirmed with endoscopy and a biopsy of the small intestine.
Treatment
Exclusion of gluten from the diet is the only available treatment for sufferers of CD. Many novel therapies are currently under investigation such as intraluminal therapies (genetic modification of wheat, pre-treatment of flours, intraluminal binding of gluten peptides and the neutralization of gluten antibodies), and therapies to reduce the immune response, such as tTG inhibitors. tTG is an attractive therapeutic target as it is responsible for the deamination of gluten derived peptides which ultimately leads to the inflammatory immune response (Sulic, et al., 2015). However, this enzyme has a varied biological role and even local inhibition may have unpredicted effects (Ciclitira, et al., 2005).
Other therapies targeting immune cells (such as bone marrow transplant or mesenchymal stem cell therapy) are promising, but due to side effects and costs compared with a gluten free diet they are not currently viable options (Schuppan, et al., 2009).
Animal Models
Several animal models have been used to try and further understand and develop novel therapies for CD.
The first animal model of CD was the Irish setter, which was found to develop partial villous atrophy and IELs when fed a wheat containing diet. However it was discovered that their gluten sensitivity was not CD4+ T-cell mediated, so it is not a model applicable to humans (Marietta, et al., 2011).
tTG blockers have been developed and initially successful when trialed in knockout mice, but as they aged they developed abnormal inflammatory responses. Long term inhibition of tTG seems to be associated with harmful complications, so reversible inhibitors may be a more viable method of minimizing the adverse effects (Kurppa, et al., 2014).
The rhesus macaque can also develop many of the features associated with CD. They show villous atrophy and as well as developing autoantibodies. This model is good for testing novel therapies as it most closely resembles CD in humans. The incidence of elevated autoantibodies is 1:125 in rhesus macaques, which is similar to the incidence of CD in the U.S. population (Marietta, et al., 2011).
The prevalence of celiac disease
General prevalence
CD is one of the most common autoimmune diseases in Europe and North America, with a case frequency averaging 1 out of 100 among Caucasians, or a prevalence of 1%. The frequency of disease is calculated by: “Seroprevalence data, typically serum tissue transglutaminase IgA antibody levels, often confirmed by positivity of endomysial antibody and in some studies biopsy” (Reilly & Green, 2012). It is estimated that only 10-15 % of people with celiac disease are actually treated, while most people never get diagnosed with the disease (Guandalini & Assiri, 2014).
By country, the frequencies of cases are not the same. Evidence suggests that some countries have higher amount of CD positive individuals in the population, while others have a lower national average. For example, in children and adolescents, Hungary has a relatively high frequency, with 1.1% of the young population estimated to have CD. Germany, on the lower end of the spectrum only has a prevalence of 0.2% (Newton & Singer, 2012). In adults, some studies suggest that these national differences can be seen as even more substantial, with Finland as the country with a high prevalence of 2.4 %, and Germany with a low prevalence of 0.3% (Reilly & Green, 2012). It is unknown if different diagnostic tactics or awareness of the disease decides the differences, or if the variance of the national frequencies is due to true differences in the prevalence of CD in the populations. Among children, Northern Africa sticks out as having a high rate of incidence of CD, particularly children of a tribe called the Sarahawi, where prevalence value is estimated at 5.6% (Reilly & Green, 2012). This is illustrated in Table 1.
Table 1: Prevalence of CD in Children and Adolescents (Source: Newton & Singer, 2012)
Country |
Prevalence |
|
Northern India |
0.32-1.00% |
|
Egypt |
0.53% |
|
Iran |
0.61% |
|
Great Britain |
0.99% |
|
Finland |
1.01% |
|
Hungary |
1.18% |
|
Sweden |
1.30-2.90% |
|
USA |
1.75-2.88% |
|
Algeria |
5.66% |
|
Demographics
CD is predominantly a Caucasian disease, where approximately 94% (Fasano, et al., 2003) of cases are people of European ethnicity or descent. However there are cases reported in South American native populations, and Northern African regions (Reilly & Green, 2012). Studies suggest that gender is also a factor, where the majority of CD positive cases are women. According to Reilly & Green (2012)“Females are diagnosed between 2-3 times more often than men, except in the young and the elderly, where the genders are more equal”. However this inequality of diagnosed cases between genders cannot with certainty be directly linked with a higher risk factor for women to have the disease. It can also be linked to different diagnostic methods, or rather differing awareness between genders. Diagnosed men however will often present with more severe symptoms and a more progressed illness.
The rising incidence of Celiac Disease
Certain studies show a trend of a rising incidence of CD cases both in national and regional aspects. CD is becoming an increasing problem in Middle Eastern, North African and South Asian regions, where it also has become an economic health burden (Reilly & Green, 2012).
There is an international trend of increasing incidence of CD. However we cannot ascertain if this is a true rise in global prevalence, as it can also be a product of higher awareness and better global healthcare. Determining if it is a true rise will need to be done on a smaller scale.
A study of childhood cases of CD in Scotland shows the trend of rising incidence is significant enough to indicate a true rise in CD cases among children and adolescents. "In the period between 1994-2009, the total number of children diagnosed with CD in Southeast Scotland was 266. The incidence ratio increased from 1.8/100 000 (95% confidence interval [CI] 1.1-2.7) in the period between 1990 to 1994, to 11.7/100 000 (95% CI 9.8-13.9) in the period between 2005-2009 (P=0.0001)” (White, et al., 2013). These numbers include patients with non-classic presentation of the disease, for example patients presenting with monosymptomatic GI disturbances. However even if we exclude these, the numbers are still significant, with a rise from 1.51/100 000(95% CI 0.91-2.38) in 1990-1994 to 5.22/100 000 (95% CI 3.98-6.75) in the period between 2005-2009 (P=0.01). This means that in Southeast Scotland, there is a provable true rise in the incidence of CD in children (White, et al., 2013). It is likely that similar experiments carried out in other regions will hold similar results.
Discussion
Gender & ethnic differences
The exact reason for the differences between genders and ethnicities is unclear. Generally speaking, males are diagnosed less frequently than females in the adult population. Among children and elderly people, the differences are smaller. If one were to do a population wide screening, Reilly and Green (2012) state that there is a smaller difference between the genders. This could potentially indicate that there is not a genetic or environmental factor that causes a higher risk of the disease for females. If one stipulates that females and males hold the same risk of developing CD, it could indicate either that males visit health care institutions less frequently than females, males are harder to diagnose correctly, or there is a difference in symptom severity that will cause a more problematic diagnosis.
If we assume that males present with more or less severe symptoms than females, this could make the disease harder to separate from other disorders that could potentially cause similar symptoms. As previously stated, it is possible that males in general have less health check-ups than females, and for sociological reasons might neglect visiting doctors, even after being troubled by symptoms. In lesser-developed countries, males are still considered to be the main source of income in the family unit, which could limit time and opportunity for men to visit doctors.
Differences in the incidence of CD between ethnicities is potentially more likely to be connected to the gene pools of different populations. As can be seen in Table 1, trends among different groups of people that have significantly higher or lower values than others. One example of this can be Sarahawi children in North Africa, a population that has a prevalence value significantly higher than average (Reilly & Green, 2012; Newton & Singer, 2012). This could indicate that among this tribe, there is a genetic predisposition to develop CD. One can also see that between western European countries, there is a potentially significant difference of incidence, with Finland being on a higher end of the spectrum, and Germany being on the lower end. This could be connected to different diets or other environmental factors and/or genetic differences between ethnicities.
Conclusion
The incidence of CD internationally is rising. It is difficult to distinguish if this is a true rise in the prevalence or due to increased global awareness of the disease. Population screenings for CD is more prevalent in western countries, and there is a significant difference in health care opportunities between developed and developing countries. Genetic and environmental variations also need to be considered when talking about the global incidence and prevalence of CD. CD has strong HLA associations and genetic links but no specific gene defect has yet been found to link phenotype with genotype. New treatments are being investigated and the improved understanding of the immune response to gluten has drastically advanced the investigation and treatment of the disease. The future is promising for suffers, but for now the maintenance of a gluten free diet is the only viable way of managing the condition.
References
Abernavoli, L., Proietti, I., Leggio, L., Ferrulli, A., Vonghia, L., Capizzi, R., Rotoli, M., Amerio, P., Gasbarrini, G., Addolorato, G., 2006. Cutaneous manifestations in celiac disease. World journal of gastroenterology, Volume 12(6), pp. 843-852.
Al-Toma, A., Verbeek, W., Hadithi, M., Von Blomberg, B., Mulder, C., 2007. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience,Gut, Volume 56(10), pp. 1373–1378.
Ciclitira, P., Johnson, M., Dewar, D. & Ellis, H. J., 2005. The pathogenesis of coeliac disease. Molecular Aspects of Medicine, Volume 26, pp. 421-458.
Fasano, A., Berti, I., Geraduzzi, T., Not, T., Colletti, R., Drago, S., Elitsur, Y., Green, P., Guandalini, S., Hill, I., Pietzak, M., Ventura, A., Thorpe, M., Kryszak, D., Fornaroli, F., Wasserman, S., Murray, J., Horvath, K., 2003. Prevalence of Celiac Diesase in At-Risk and Not-At-Risk groups in the United States. Archives of Internal Medicine, Volume 163, pp. 286-292.
Guandalini, S. & Assiri, A., 2014. Celiac Disease A Review. Pediatrics, Volume 168(3), pp. 272-278.
Kagnoff, M., 2007. Celiac disease: pathogenesis of a model immunogenetic disease. The Journal of Clinical Investigation, Volume 117(1), pp. 41-49.
Kagnoff, M., 2012. Introduction: Celiac Disease. Seminars in Immunopathology, Volume 34(5), pp. 471-472.
Kurppa, K., Hietikko, M,. Sulic, A., Kaukinen, K., Lindfors, K., 2014. Current status of drugs in development for celiac disease. Expert Opinion on Investigational Drugs, Volume 23(8), pp. 1079-1091.
Marietta, E., David, C. & Murray, J., 2011. Important Lessons Derived from Animal Models of Celiac Disease. International Reviews of Immunology, Volume 30, pp. 197-206.
Mouterde, O., Dumant, C., Mallet, E., 2013. Symptoms of Celiac Disease in childhood. Pathologie Biologie, Volume 61, pp. 53-55.
Newton, K. & Singer, S., 2012. Celiac disease in children and adolescents: special considerations. Seminars in Immunopathology, Volume 34(5), pp. 479-496.
Reilly, N. & Green, P., 2012. Epidemiology and clinical presentations of celiac disease. Seminars in Immunopathology, Volume 34(6), pp. 473-478.
Rosén, A., Sandstrom, O., Carlsson, A., Hogberg, L., Olén, O., Stenlund, H., Ivarsson, A., 2014. Usefulness of Symptoms to Screen for Celiac Disease. Pediatrics, Volume 133(2), pp. 211-217.
Samasca, G., Sur, G., Lupan, I., Tilinca, M., Deleanu, D., 2014. Celiac disease as an autoimmune condition. Central European Journal of Immunology, Volume 39(3), pp. 396-399.
Schuppan, D., Junker, Y. & Barisani, D., 2009. Celiac Disease: From Pathogenesis to Novel Therapies. Gastroenterology, Volume 137(6), pp. 1912-1933.
Sulic, A., Kurrpa, K., Rauhavirta, T., Kaukinen, K., Lindfors, K., 2015. Transglutaminase as a therapeutic target for celiac disesae. Expert Opinion on Therapeutic Targets, Volume 19(3), pp. 335-348.
White, L., Merrick, V., Bannerman, E., Russell, R., Basude, D., Henderson, P., Wilson, D., Gillett, P., 2013. The Rising Incidence of Celiac Disease in Scotland. Pediatrics, Volume 132(4), pp. 924-931.
Woodward, J., 2010. Coeliac disease. Medicine, Volume 39(3), pp. 173-177.