Celiac Disease: Incidence and Causes
Celiac Disease, hereby CD, is a disease that is triggered by the ingestion of gluten (Guandalini & Assiri, 2014). It results in a disorder in the small intestine due to an inflammatory reaction to the gluten molecule (Guandalini & Assiri, 2014). Approximately 1 % of the European and USAs population suffers from CD (Guandalini & Assiri, 2014). The name celiac derives from the Greek "koiliakos", meaning "belly" (Woodward, 2010). Only 10% to 15% of this affected population have been diagnosed and treated (Guandalini & Assiri, 2014). There is a wide array of clinical signs for celiac disease, many of them are not directly related to the gastrointestinal tract and this may therefore delay the diagnosis (Kagnoff, 2012). This is a disease that can affect people in any age and the treatment is a lifelong gluten-free diet.
Contents
Signs and symptoms
The primary reaction is the inflammation and damage of the small intestine and as a result the absorbance of important nutrients such as iron, folate, vitamin B12, calcium, proteins, fats and fat-soluble vitamins suffers. This causes secondary reactions as anemia, osteoporosis and abnormal bleeding. Celiac disease is frequently found together with other autoimmune diseases, for example type 1 diabetes mellitus, autoimmune thyroiditis, autoimmune hepatitis, dermatitis herpetiformis and autoimmune alopecia (Schuppan, et al., 2009). Physicians should be aware for atypical cases, so as to improve diagnosis and avoid complications (Ciclitira, et al., 2005; Mouterde, et al., 2013). Symptoms mainly manifest themselves in the gastrointestinal tract, but there are many extra-intestinal symptoms too.
Gastrointestinal tract
Abdominal pain and diarrhea are the most common symptoms. Rarer symptoms are electrolyte disturbance, hypotension and lethargy, these symptoms are particular when there is a late diagnosis (Guandalini & Assiri, 2014). Younger patients (children under 2 years) usually show more symptoms such as diarrhea or malabsortive manifestations, which are considered to be the more "classical" symptoms (Reilly & Green, 2012). Older children and adults are more likely to show symptoms that are not directly connected to the gastrointestinal symptoms (Reilly & Green, 2012).
Patients that have symptomatic celiac disease for a long time without treating it are at an higher risk of developing cancer as enteropathy-associated T-cell lymphoma and small bowel adenocarcinoma of the gastrointestinal tract (Schuppan, et al., 2009). Refractory celiac disease can develop in 5-10% of adults with undetected and untreated celiac disease for a long period. It occurs when your intestine fail to heal. These patients do not respond to or experience a relaps even though they are on a strictly gluten-free diet. Refractory celiac disease is classified as Type 1 and Type 2 (Schuppan, et al., 2009).
Extra-intestinal manifestations
CD presents with a wide variety of symptoms such as tiredness, nausea, reduced appetite, stomach pains, vomiting, abdominal gas, bloating, hard or loose stools and weight loss (Rosén, et al., 2014). CD can affect the growth of children in a negative way, and may also delay puberty (Newton & Singer, 2012). The disease may also affect the bone mineral content and cause osteoporosis, which increase the risk of bone fractures (Kurppa, et al., 2014). Other symptoms to celiac disease can be reproductive disorder, splenic hypofunction, dental enamel defects, cardiac problems and liver damage (Kurppa, et al., 2014).
Symptoms such as skin disease have also been described in CD patients, and increased rates of psoaris have been observed (Abernavoli, et al., 2006; Samasca, et al., 2014). An associated condition is Dermatitis Herpertiformis, which causes itchy blistering skin, mostly effecting knees, elbows, back, buttocks and occasionally also within the mouth (Ciclitira, et al., 2005). Although it is rare for patients with coeliac disease have dermatitis herpertiformis, all patients with dermatitis herpertiformis have some degree of enteropathy (Woodward, 2010).
What causes Celiac Disease?
Gluten, a protein found in barley, wheat and rye, can cause celiac disease. In some people who are exposed to it, an enzyme called tissue transglutaminase (tTG) alters the gluten in such a way that it causes an immune response, which leads to inflammation of the gut. Small projections known as villi line the gut and are important in increasing the surface area available for nutrient absorption. In CD, these villi are flattened and destroyed, preventing the normal absorption of nutrients.
Pathogenesis
CD occurs as a result of environment influences in genetically predisposed individuals (Guandalini & Assiri, 2014). According to Schuppan (2009) CD can be characterized by (1) a defined trigger, such as gluten proteins found in wheat and other cereals, (2) The presence of human leucocyte antigen (HLA) class II haplotypes DQ2 or DQ8, and (3) the production of autoantibodies to the enzyme tissue transglutaminase (tTG).
1) CD is triggered by proteins in cereal grains such as wheat, barley and rye. Gluten is the term used to describe the disease-activating proteins in these cereals. Gluten comprises of two major protein types, gliadins and glutenins, both of which possess disease-activating peptides rendering them resistant to complete digestion (Kagnoff, 2007).
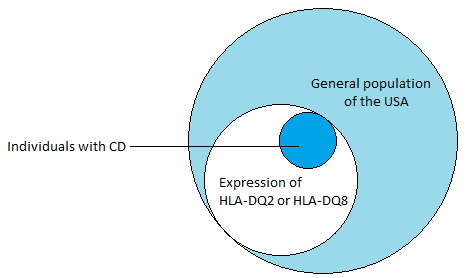
2) The presence of MHC class II HLA-DQA alleles, HLA-DQ2 and HLA-DQ8, is the most important genetic susceptibility factor contributing about 40% (see Figure 1) to the risk of developing CD (Schuppan, et al., 2009). These molecules are expressed on by antigen presenting cells in the lamina propria of the small intestine, and are responsible for presenting gliadins (gluten proteins) to CD4+ T cells, which triggers an inflammatory immunological response (Guandalini & Assiri, 2014). tTG has a high avidity for the gluten peptides and is crucial in the deamination of gluten epitopes (Ciclitira, et al., 2005). tTG works by causing the deamination of glutamine residues found in gluten peptides (Kagnoff, 2012). Once the deaminated peptide is bound to the HLA-DQ2 or HLA-DQ8, the complex can activate the CD4+ T cells resulting in the inflammation through the secretion of IFN-ɣ (see Figure 3). IFN-ɣ is thought to play a key role in the downstream regulation of mucosal damage as it has been shown the neutralization of IFN-ɣ prevents mucosal damage (Kagnoff, 2007).
(3) The final characteristic of CD is the presence of autoanitbodies which are generated as part of the immunological response to enzyme tissue transglutaminase (tTG). These autoantibodies (such as anti-tTG IgA and antiendomysium IgA) are highly specific to the condition and can be used for validation and diagnostic purposes.
|
Diagnosis
Treatment
Animal Models
The prevalence of celiac disease
General prevalence
Demographics
The rising prevalence of Celiac disease
Discussion
Gender & ethnic differences
Conclusion