Itt írjon a(z) diabetes_virus_eng-ról/ről
Viral Triggers for Diabetes Mellitus
By: Rivka Bobrovsky, Nicolas Mannarino, Amrita Marath
Supervisor: Dr. Kiss David Sandor
University of Veterinary Medicine Budapest, Hungary 2022
table of contents:
1. INTRODUCTION
2. PHYSIOLOGICAL BACKGROUND
2.1 DIABETES MELLITUS TYPE I
2.2 DIABETES MELLITUS TYPE II
3. VIRAL BACKGROUND
3.1 ENTEROVIRUSES: COXSACKIEVIRUS TYPE B4 (CVB4)
3.2 SARS-COV-2
4. PATHOPHYSIOLOGY
4.1 ENTEROVIRUS TYPE ASSOCIATED WITH DIABETES MELLITUS TYPE I
4.2 SARS-COV-2 ASSOCIATED WITH DIABETES MELLITUS TYPES I AND II
5. PREVENTION AND TREATMENT
6. CONCLUSION
7. BIBLIOGRAPHY
8. OTHER REFERENCE MATERIALS
1. Introduction
Diabetes is a long-term condition that negatively impacts millions of people around the world. It is estimated that in 2019 the global prevalence of diabetes was 9.3%, totaling 430 million affected individuals (Saeedi et al, 2019).
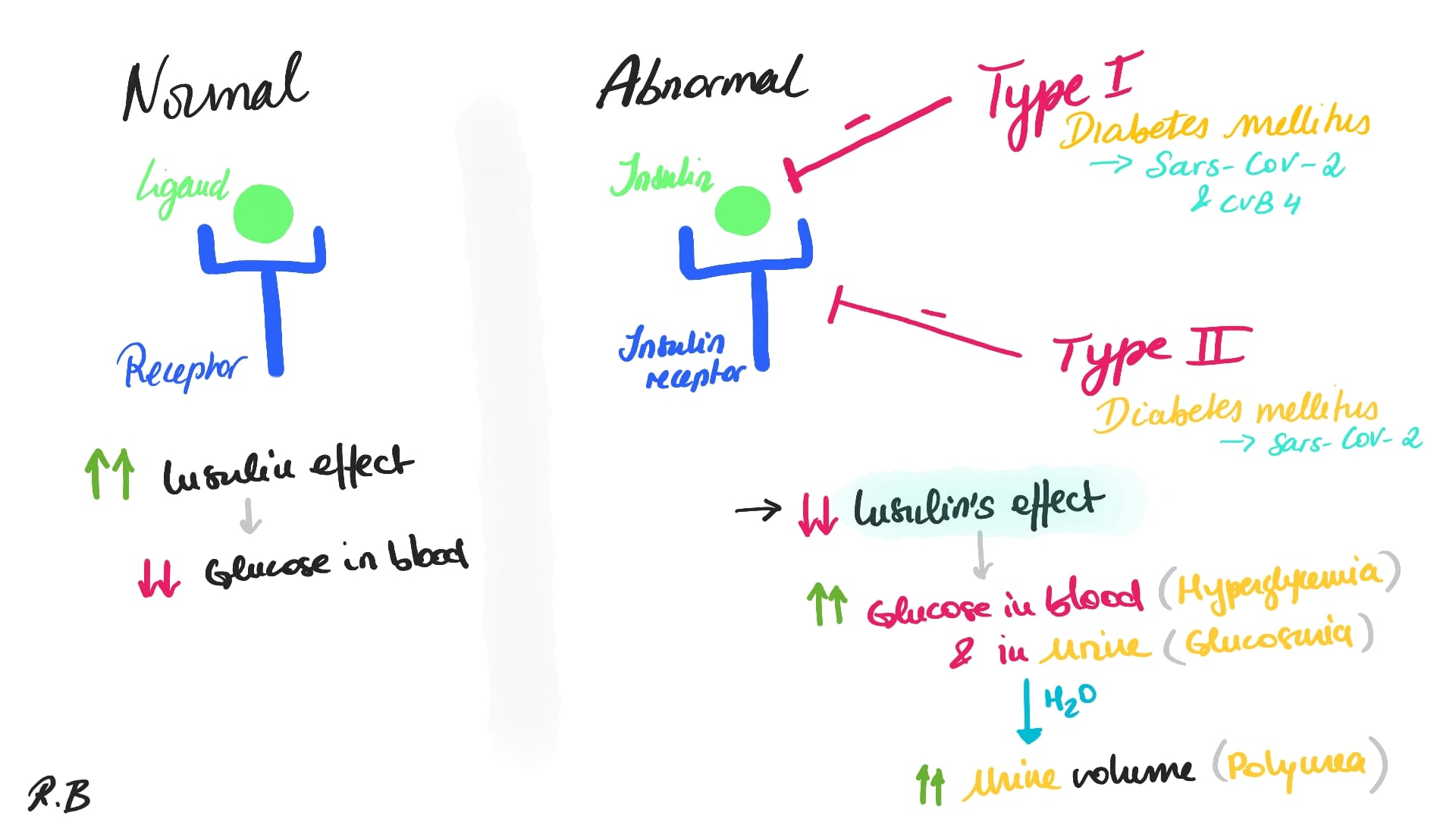
According to the American Diabetes Association (2007), the diabetes mellitus is characterised by hyperglycemia as the result of either deficiencies in insulin secretion (Type 1) or a resistance to the effects of insulin in conjunction with poor insulin secretory response (Type 2). Following the onset of the Covid-19 pandemic, several studies have shown that diabetes mellitus is a major comorbidity factor for the SARS-CoV-2 virus, increasing risk of in-hospital death by a factor of 2.85 (Zhou et al, 2020). This is especially troubling due to a growing foundation of anecdotal evidence suggesting that the SARS-CoV-2 virus is correlated with the development of diabetes (Sathish et al, 2020).
There are two types of viruses that can trigger diabetes mellitus, namely: (i) the enteroviruses, especially Coxsackievirus type B4 (CVB4), which can only trigger Type I diabetes and (ii) the SARS-CoV-2 which can trigger both diabetes types I and II (hyperglycemia) as recent studies have shown.
After a quick overview of the physiological background of both diabetes mellitus type 1 (2.1) type 2 (2.2) and the viral background of the enteroviruses (3.1) and SARS-CoV-2 (3.2), we will present our findings on the relationship / correlation between these viruses (environmental factors) and the diabetes mellitus (4), as well as how they can prevent (if possible) and treatments in case of infection (5).
|
2. Physiological Background
Diabetes mellitus is caused either by a lack of insulin production by the pancreas “Diabetes mellitus type 1” (2.1) or a lack of insulin response by the body's cells “Diabetes mellitus type 2” (2.2). As it is commonly known, Insulin is a hormone that aids in the transport of glucose from diet into cells for energy production and thus allowing to keep the glucose concentration at a physiological level to avoid hyperglycemia.
2.1 Diabetes Mellitus Type I
Type 1 of diabetes mellitus (T1D) is an autoimmune disease in which pancreatic beta cells of the Islet of Langerhans are selectively and progressively destroyed, leading to high concentration of glucose in the circulation. As result, animals suffering from T1D must administer exogenous insulin throughout their lives. T1D can be caused by the complex interaction between genetic, immune, and environmental factors (Dalzochio, Thaís, et al., 2019). Researchers have shown that enteroviruses are thought to be the most important environmental component in this disease (cf. Richardson SJ, Morgan NG, 2018; Buschard, 2011).
2.2 Diabetes Mellitus Type II
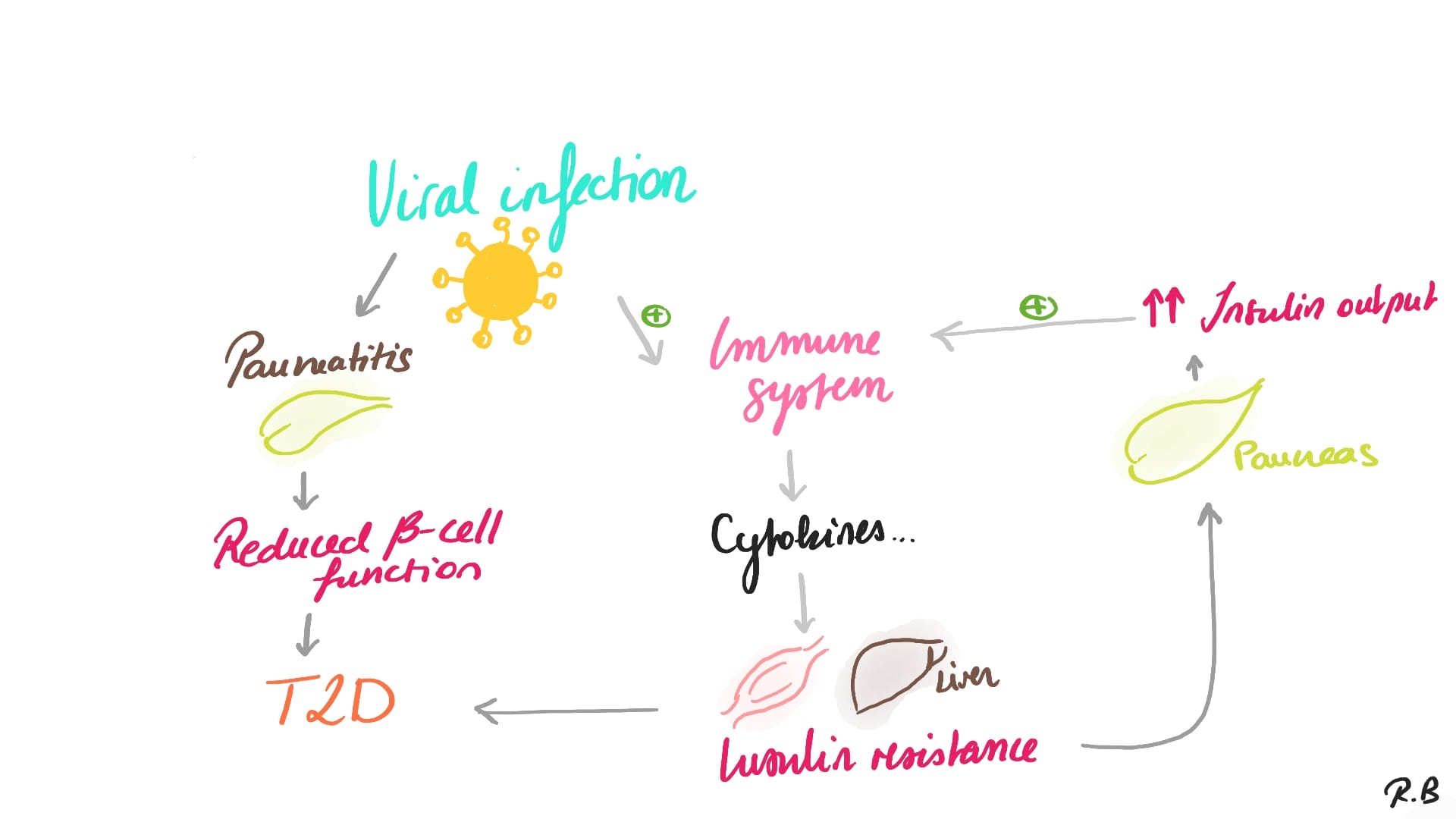
Insulin resistance, a disease in which cells do not respond appropriately to insulin, is the starting point for type 2 diabetes (T2D). A shortage of insulin may occur as the condition advances. Previously, this condition was known as "non-insulin-dependent diabetes mellitus" or "adult-onset diabetes." Although T2D is more common in older persons, an increase in the incidence of obesity among youngsters has resulted in an increase in the number of instances of T2D in children. A combination of excessive body weight and insufficient exercise is the most typical cause (cf. Ettinger, S.J, Feldman, E.C, 2005).
3. Viral background
3.1 Enteroviruses: Coxsackievirus type B4 (CVB4)
Human enteroviruses (HEV), particularly those belonging to the Coxsackievirus B family (CVB), are known to infect the beta cells resulting in the progressively and systematically destruction of the beta cells of the Islet of Langerhans, which in return entails a significant reduction in glucose-induced insulin production and thus causing diabetes mellitus type 1 (T1D). Indeed, studies have shown that isolated islets can be successfully infected in vitro with a variety of the EV-B family members (CVBs and Echoviruses), many of which have been linked to the diabetes mellitus type 1 (Richardson SJ, Morgan NG, 2018; Wing-Chi G Yeung et al., 2011). HEVs' islet tropism has also been established in vivo in the pancreas of newborns who died after contracting a deadly CVB infection and in the pancreatic of T1D patients (Richardson SJ, Morgan NG, 2018; Wing-Chi G Yeung et al., 2011).
The enteroviruses don’t bind to the same type of protein present in the beta cells, but rather to different type of receptors. Many viruses linked to diabetes mellitus type 1 employ the Coxsackie and Adenovirus Receptor (CAR) as an entrance vehicle. Studies have shown that specific isoform of CAR with a unique C-terminal PDZ binding domain (CAR-SIV) is specifically and strongly produced within beta cells in epidemiological investigations (Richardson SJ, Morgan NG, 2018). According to research, CAR-SIV is found in significant concentrations on insulin secretory granules. During exocytosis of insulin, the extracellular domain of CAR-SIV will be shown on the plasma membrane's external face, ready to bind to enteroviruses. Thus, the virus would be delivered inside the cell during subsequent endocytosis of the granule membrane for recycling, where it may commence infection (Richardson SJ, Morgan NG, 2018).
3.2 SARS-CoV-2
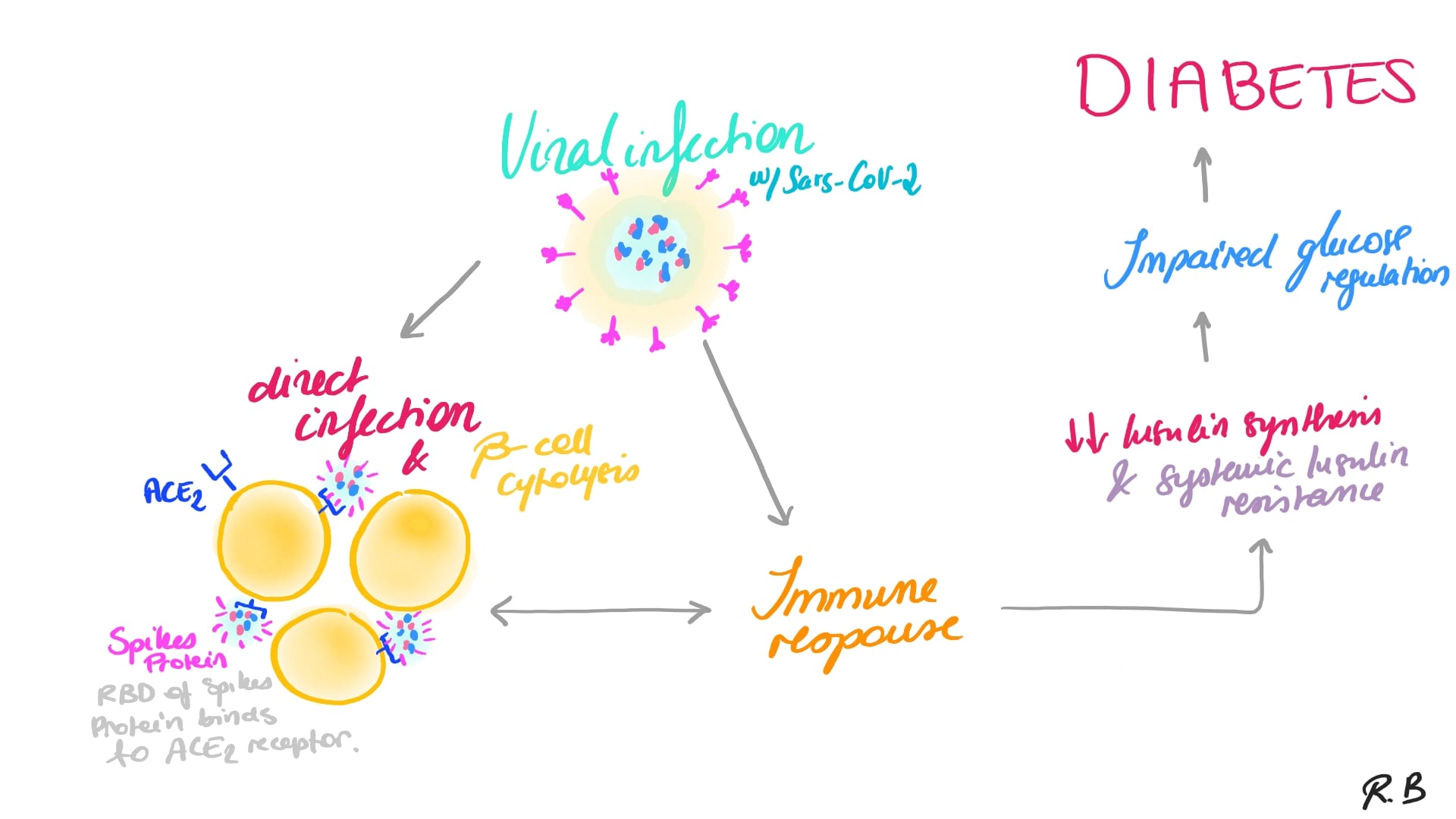
SARS-CoV-2 is a member of the Coronavirus family, which is a broad family of viruses. People and certain animals can be infected by these viruses. SARS-CoV-2 was first discovered infecting humans in 2019 in three persons who had pneumonia and were linked to a Wuhan cluster of acute respiratory sickness cases (Yuki et at, 2020). Droplets emitted when an infected person coughs, sneezes, or speaks transfers the virus from person to person (Yuki et al, 2020). It can also be transferred by contacting a virus-infected surface and then touching one's lips, nose, or eyes, however this is rare (Yuki et al, 2020). COVID-19 is being studied, as is the prevention of SARS-CoV-2 infection. Coronavirus 2 is also known as severe acute respiratory syndrome coronavirus 2. In nature, all the structural properties of the novel SARS-CoV-2 virus particle are seen in related coronaviruses (cf. Reiterer et al., 2021; Bernard et al., 2020 Hollstein et al., 2020; Lim et al., 2020).
4. Pathophysiology
Studies have shown that there is a strong correlation between the enteroviruses – especially the Coxsackievirus type B4 (CVB4), and diabetes type 1 (4.1) and the SARS-CoV-2 and the appearance of diabetes mellitus type 1 or type 2 (4.2).
4.1 Enterovirus type associated with diabetes mellitus type I
The tropism of enteroviruses for beta cells is likely to be driven by at least two factors: first, these cells express receptors required for virus binding and subsequent internalization, and second, they contain specific host factors that the virus can hijack to facilitate infection, replication, and possibly persistence (cf. Richardson SJ, Morgan NG, 2018).
Following a HEV infection, the pancreas is primed to respond to the possibility of a local viral infection by systemic release of interferons. Usually, this will result in the activation of an antiviral defense system, which will prevent the beta cells from developing a long-term and productive infection. The infection is eradicated, and the host triumphs (Richardson SJ, Morgan NG, 2018; Massilamany et al, 2016). In other words, enteroviruses may initiate or accelerate the pathogenic events contributing to T1D through several mechanisms (Massilamany et al, 2016; contra: Buschard, 2011). The beta cells of Islet of Langerhans can be killed either via (i) the enterovirus's cytolysis induction, which happens immediately and / or through (ii) a less aggressive infection could also trigger an inflammatory response in the islets, resulting in subclinical beta cell loss and subsequent antigen release, activating autoreactive T cells. Cross-reactive T cells, which occur when viral and host antigens share the same antigenic determinants, could also be generated (cf. Massilamany et al, 2016; contra: Buschard, 2011).
However, in some patients it was shown that enterovirus use crucial host components to establish a productive and lytic infection in some individuals who have a compromised antiviral defense. This can result in the release of free virus and/or antigens specific to viral and the beta cells. This damage may activate islet autoreactive immune cells in individuals who are genetically prone to T1D. A prolonged persistent infection may occur if the host's antiviral defense systems only partially block the viral replication. Researchers have shown that the so-called “persistent infections” are linked to viral genome 5′UTR deletions and the generation of dsRNA. The latter can trigger an increased interferon signature in cells by activating host pathogen recognition receptors (PRRs), such as Mda5 (encoded by IFIH1). As a result, HLAI will be upregulated, and beta cell and viral antigens will be better presented at the cell surface. In 'at-risk' persons, this could lead to auto-reactive destruction (cf. Richardson SJ, Morgan NG, 2018).
4.2 SARS-Cov-2 associated with diabetes mellitus types I and II
Recent studies demonstrated that SARS-CoV-2, the virus causing COVID-19 infections, can trigger diabetes mellitus (hyperglycemia) since SARS-Co-V2 receptors were found in the beta cells of the Islet of Langerhans (cf. Sasaki et al. 2022; Yano et al, 2022; Reiterer et al., 2021; Bernard et al., 2020; Hollstein et al., 2020; Lim et al., 2020).
Reiterer and his team showed that Adiponectin levels, which are secreted by adipocytes – a well-known homeostatic factor for regulating glucose levels, were found to be lower in COVID-19 patients in a glucoregulatory hormone screen (Sasaki et al., 2022; Reiterer et al., 2021; see also: Bernard et al., 2020; Hollstein et al., 2020; Lim et al., 2020). Furthermore, SARS-CoV-2 infected hamsters had a significant antiviral gene expression program in their adipose tissue, as well as lower adiponectin expression. Moreover, Reiterer and his team showed that SARS-CoV-2 can also infect adipocytes. These findings imply that SARS-CoV-2 may cause adipose tissue malfunction, which leads to insulin resistance and poor outcomes in COVID-19 patients (cf. Reiterer et al., 2021; Bernard et al., 2020).
|
|
5. Prevention and treatment
To prevent or at least reduce the risk of getting diabetes mellitus following a viral infection, one would think that the best way to do it, is by getting the vaccines against both those viruses. This may be true for enteroviruses (Kondrashova, Hyöty, 2014), but not so much for SARS-CoV-2 (Sasaki et al., 2022; Yano et al, 2022). Indeed, very recent studies have shown that the vaccine for the coronavirus disease (COVID-19) may worsen or even cause the diabetes mellitus. The findings were based on two different cases about two Japanese woman’s who, after getting vaccinating against SARS-CoV-2 mRNA, showed high concentration of glucose in their blood plasma, one a few weeks after her first doses (Yano et al., 2022) and the other one a few weeks after her second dose (Sasaki et al., 2022). In this case, today’s available treatment seems to be the better and or only option.
Several researchers also suggest that, following these results, a possible prevention and therapeutic strategy would be to use, in combination with anti-viral therapies, inhibitors against DNA methylase transferase, a protein responsible for the hypermethylation of the genome and silencing of Pdx1. Indeed, it was demonstrated that this class of inhibitors reinstated PDX1 expression and glucose tolerance in diabetic mice. Several of these inhibitors have already been licensed for clinical use in cancer treatments, which could speed up their application in these case (Bernard et al., 2020).
6. Conclusion
In conclusion, the presence of specific proteins (receptors) in the beta cells of Islet of Langerhans make it possible to the enteroviruses, especially the Coxsackievirus type B4 (CVB4) and/or the SARS-CoV-2 to trigger subsequently sever long-term or long-lasting diseases, such diabetes mellitus. As we saw in our present study, the former will cause only diabetes type 1, whereas the latter will be able to cause either diabetes mellitus type 1 or the type 2. Meaning these viruses will either decrease the efficiency of the insulin or increase its resistance to insulin and thus leading to hyperglycemia, which in return may cause other serious health issues such vision problems, foot ulcers, kidney failure, cardiovascular disease etc. Therefore, it is crucial to promote prevention by getting the enterovirus vaccines (but not so much against COVID-19, since the specific vaccine can cause or worsen diabetes mellitus in certain patients, as some recent study cases from Japan and China have shown) and, in case of infection, treat the patient immediately with the appropriate available treatment.
7. Bibliography
American Diabetes Association (2007): Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1: S42–S47. https://doi.org/10.2337/dc07-S042
Bernard H, Teijeiro A, Chaves-Pérez A, Perna C, Satish B, Novials A, Wang JP, Djouder N (2020): Coxsackievirus B Type 4 Infection in ß Cells Downregulates the Chaperone Prefoldin URI to Induce a MODY4-like Diabetes via Pdx1 Silencing. (Cell Reports Medicine). DOI: 10.1016/j.xcrm.2020.100125 Buschard, K (2011): 'What causes type 1 diabetes? Lessons from animal models', A P M I S. Acta Pathologica, Microbiologica et Immunologica Scandinavica, vol.119, no. Suppl. 132, pp. 1-19. https://doi.org/10.1111/j.1600-0463.2011.02765.x
Dalzochio T, Nienov OH, Wannmacher C, Berlese DB, Feksa LR (2019): "Effects of bovine enterovirus and type 1 diabetes on liver and kidney pyruvate kinase activity in an animal model." Acta Scientiarum. Biological Sciences 41: e43765-e43765.
Hollstein T, Schulte DM, Schulz J, Glück A, Ziegler AG, Bonifacio E, Wendorff M, Franke A, Schreiber S, Bornstein SR, Laudes M (2020): Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020 Oct 2(10):1021-1024. doi: 10.1038/s42255-020-00281-8. Epub. PMID: 32879473.
Kondrashova A & Hyöty H (2014) Role of Viruses and Other Microbes in the Pathogenesis of Type 1 Diabetes, International Reviews of Immunology, 33:4, 284-295, DOI: 10.3109/08830185.2014.889130 https://www.tandfonline.com/action/showCitFormats?doi=10.3109%2F08830185.2014.889130 Lim S, Bae JH, Kwon HS, Nauck MA (2020): COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021 Jan;17(1):11-30. doi: 10.1038/s41574-020-00435-4. Epub 2020 Nov 13. PMID: 33188364; PMCID: PMC7664589.
Massilamany C, Koenig A, Reddy J, Huber S, Buskiewicz I (2016): Autoimmunity in picornavirus infections. Current Opinion in Virology, 16(1), 8-14. doi: 10.1016/j.coviro.2015.10.004
Reiterer M, Rajan M, Gomez-Banoy N, Schenck EJ, Safford MM, Lo JC (2021): Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2, Cell Metabolism. https://www.cell.com/cell-metabolism/fulltext/S1550-4131(21)00428-9#secsectitle0020
Richardson SJ, Morgan NG (2018): Enteroviral infections in the pathogenesis of type 1 diabetes: new insights for therapeutic intervention. Curr Opin Pharmacol. 2018 Dec;43:11-19. doi: 10.1016/j.coph.2018.07.006. PMID: 30064099; PMCID: PMC6294842, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6294842/
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala A A, Ogurtsova K, Shaw JE, Bright D, Williams R (2019): Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Elsevier; Diabetes Research and Clinical Practice I57: 107843
Sasaki H, Itoh A, Watanabe Y, Nakajima Y, Saisho Y, Irie J, Meguro S, Itoh H (2022): Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J Diabetes Investig. Epub ahead of print. PMID: 35088548. https://pubmed.ncbi.nlm.nih.gov/35088548/
Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P (2020): Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab [Internet]. [cited 2022 Mar 27]; Available from: https://dompubs.onlinelibrary.wiley.com/doi/abs/10.1111/dom.14269
Yano M, Morioka T, Natsuki Y, Sasaki K, Kakutani Y, Ochi A, Yamazaki Y, Shoji T, Emoto M. (2022): New-onset Type 1 Diabetes after COVID-19 mRNA Vaccination. Intern Med. 1197-1200. doi: 10.2169/internalmedicine.9004-21 PMID: 35135929.
Yeung WG, Rawlinson WD, Craig ME (2011): Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies BMJ 342; d 35 doi:10.1136/bmj.d35, https://www.bmj.com/content/342/bmj.d35
Yuki K, Fujiogi M, Koutsogiannaki S (2020): COVID-19 pathophysiology: A review. Clinical Immunology. 215(108427): 1-7; https://doi.org/10.1016/j.clim.2020.108427
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z (2020): Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 395(10229):1054–62.
8. Other Reference Materials
Ettinger SJ, Feldman EC (2005): Textbook of Veterinary Internal Medicine (6th edition, volume 2) Elsevier Saunders