Toxic Effects of Metals on the Hypothalamus
Contents
Metal toxicity
Metal toxins including cadmium, copper, mercury and lead toxicity effects on the hypothalamus will be discussed in this essay. The general background of hypothalamus and blood brain barrier background are described to help with a better understanding of this aspect of the brain and how it protects itself from harm. Hypothalamus plays an important role in homeostasis affecting behavioral and physiological activities through regulation of autonomic nervous system and pituitary gland hormone secretions control (Klein, 2020).
The Hypothalamus
The hypothalamus is an area of the diencephalon that forms the floor of the third ventricle and consists of the optic chiasma, tuber cinereum, mamillary bodies and the median eminence. The hypothalamus coordinates the activity of the pituitary gland through the secretion of peptides and amines. The latter influences the pituitary gland to produce tropic hormones which in turn influence the secretion of hormones by peripheral target endocrine tissues or hormones that directly act on tissues (Klein, 2020). As illustrated in Figure 1, the location of the hypothalamus and pituitary gland in the brain is represented.
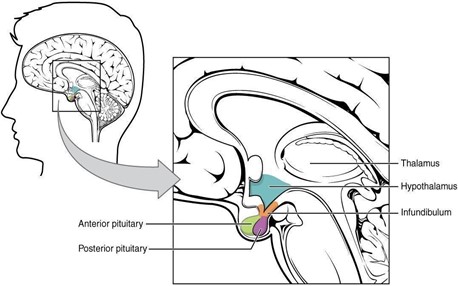
Figure 1. Hypothalamus and Pituitary gland
Blood Barrier of the Brain
Blood brain barrier (BBB) is vital for brain protection as blood can carry variable compositions of nutrients, toxins from the environment and factors from exercising, illness and age which can all affect the brain if there is no selective permeability. This highly selective permeability is provided by the tight junctions blocking the intracellular clefts in the brain capillaries allowing only small, uncharged, lipid soluble and unbound-plasma proteins to pass through such as alcohol, oxygen and carbon dioxide (Klein, 2020).
However, this can be overcome by other mechanisms allowing the entry of toxins such as cadmium which can enter via nasal mucosa or olfactory pathways. It crosses the BBB via a zinc transporter reaching into peripheral and central neurons leading to toxicity. Therefore, the toxins accumulation can happen via multifactorial mechanisms inducing metal toxicity in the brain affecting both physiological and behavioral functions of the hypothalamus (Tobwala et al., 2014).
Cadmium
Cadmium is one of the most significant toxic metals because of its utilization in industrial and agricultural practices leading to the environmental accumulation of the chemical. Cadmium in water and soil can reach human by transferring through food chain. It particularly harms the hypothalamus as it can cross through the blood brain barrier (Romero et. al, 2011).
Presently, the exposure of environmental cadmium has decreased as per the public health measures through public information and appropriate disposal of electronic waste. The study has shown that cadmium is an endocrine disrupter for humans and other species including rats, pigs, fish and frogs. This heavy metal especially affects the hypothalamic-pituitary-gonadal axis (Lafuente, 2013).
A study was conducted on male rats during puberty or adulthood by subcutaneously and orally administering cadmium for 30 days. The study supported the age-dependent effects of cadmium on hypothalamic biogenic amines including dopamine, norepinephrine, and serotonin levels. In the anterior, medio-basal and posterior hypothalamus showed an increased in norepinephrine levels on male rats that had water containing cadmium chloride of 50 ppm for 30 days. However, catecholamine levels dropped in the anterior and medio-basal hypothalamus, but its level is increased in the posterior region. As for rats treated with cadmium chloride subcutaneously with dosage between 0.5 and 1.0mg/kg every 4 days, the dopamine levels dropped in the anterior and medio-basal hypothalamus (Lafuente, 2013).
Furthermore, cadmium exposure affects glutamate concentration as it inhibits the sodium dependent glutamate/aspartate transporters in astrocytes and synaptic dysfunction leading to drop in glutamate sodium dependent uptake. Consequently, the decrease in glutamate levels in the hypothalamus and the disruption effects on transferring receptor gene expression can have a negative impact on the neonatal brain in the case of maternal cadmium exposure (Lafuente, 2013). The study showed alterations in behaviour and cognitive functions in pubertal male rats that had cadmium in their diet. Acetylcholinesterase activity increased whilst the sodium, potassium and ATPase activity significantly dropped leading to the rats having anxiety-like behaviour. Cadmium toxicity on the hypothalamus is partly from the cellular death stimulated by oxidative stress causing disruptions to intracellular homeostasis and mitochondrial dysfunction (Lafuente, 2013). Hence, cadmium increases the levels of reactive oxygen species.
However, anti-oxidants can be used as a defense against cadmium toxicity. Melatonin secreted by the pineal gland is especially effective as an anti-oxidant against cadmium as it has a stable cell membrane which can form a stable complex with heavy metals. The study affirms the therapeutic effects of the exogenous anti-oxidants supplementation against the hypothalamus neurotoxicity caused by cadmium (Romero et al., 2011).
Copper
Copper plays a vital role in physiological processes as many enzymes need copper as a co-factor and several other biochemical processes. However, it can be toxic at higher concentrations than normal. Copper can be found in farmlands and water worldwide due its use in agriculture and mining practices. Oxidative stress can be caused by copper leading to release of reactive oxygen species which lead damage in DNA and cells as discussed earlier with cadmium effects (Liao et al., 2019). Copper can cross through blood-brain barrier and accumulate in hypothalamus disruption hormone that would normally be regulated by hypothalamus. Eventually, without any regulation and high oxidative stress, hypothalamus will be damaged. However, there are anti-oxidant systems in place including superoxide dismutase and catalase which are the enzymes acting against the oxidative stress. Superoxide dismutase eliminate oxyradicals by catalysing superoxide radicals to hydrogen peroxide whilst catalase in peroxisomes removes hydrogen peroxide. Hence, these enzymes can be used for measurement between the antioxidation ability versus the reactive oxygen species damage (Liao et al, 2019).
Mercury
Mercury (Hg) is present in the environment mainly in three chemical forms: elemental Hg vapor, inorganic Hg salts, and organic Hg compounds, such as methylmercury (MeHg) (Clarkson, 2002). Methylmercury is naturally produced mostly in the aquatic environment by the methylation of inorganic mercury, in a process that takes place in aquatic microorganisms (Farina and Aschner, 2019). It is a highly toxic environmental pollutant that predominantly impacts the CNS, leading to neurological changes. One of the early symptoms of MeHg poisoning is the loss of body weight and appetite (Ferrer et al., 2018), and is regulated by multiple mechanisms. As such, Ghrelin is the only recognized peripheral hormone that stimulates food intake in the hypothalamus (Ferrer et al., 2021). In the hypothalamus, ghrelin mediates its actions by activating AMP-activated protein kinase alpha (AMPKα), leading to changes in mitochondria, and the regulation of neuropeptides that modulate energy homeostasis (López et al., 2008)
In a recent study, Ferrer et al., 2021 studied the ghrelin response in mice exposed to MeHg via drinking water. After 15 and 30 days of MeHg exposure, ghrelin’s effects on body weight and food intake were analysed. Mice were injected twice with 1 μg ghrelin/g body weight and it was found that MeHg enhanced the ghrelin-induced body weight gain in males, whereas in females it mitigated ghrelin’s effect (Ferrer et al., 2021). It was also observed that MeHg affected the expression of hypothalamic neuropeptides that regulate food intake and body weight in a gender and dose dependent manner in hypothalamic neuronal mouse cell line GT1-7 (Ferrer et al., 2018). In vitro observations were made whereby 8-week-old mice were exposed to MeHg at different concentrations in drinking water. After 30 days of exposure, there were no changes in body weight. However, MeHg treated males showed a significant reduction in fat depots (Ferrer et al., 2018).
Mercury was reported to inhibit the specific binding of luteinizing hormone-releasing hormone (LHRH) to the hypothalamic membrane (Chan et al., 1987). In fish, the hypothalamic–pituitary–gonadal (HPG) axis plays a key role in the normal development of the reproductive system. Different hormones are secreted by the hypothalamus, pituitary, and gonad to regulate reproductive activity as shown in figure 2. The hypothalamus controls the synthesis and release of gonadotropin-releasing hormone (GnRH) which acts on the pituitary, stimulating the release of luteinizing hormone and follicle stimulating hormone, which in turn act on the ovaries and testes, triggering gonad maturation, gametogenesis, and steroidogenesis (Meier et al., 2011; Zhang et al., 2016). It has also been demonstrated that waterborne inorganic Hg exposure caused histological damage in the gonads of zebrafish, induced oxidative stress in the testis, and altered sex hormone levels by disrupting the transcription of related HPG-axis genes, which could hinder the reproduction of fish (Zhang et al., 2016).
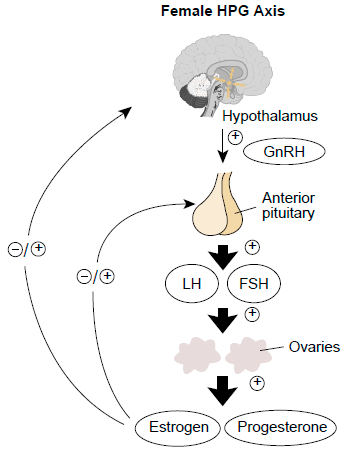
Figure 2. Hypothalamic-Pituitary-Gonadal axis
Additionally, the anterior pituitary and hypothalamus are the most important sites for estrogenic action into the brain (Kalra and Kalra, 1983). In the absence of ovarian function, the Hypothalamic–pituitary reproductive axis becomes potentially affected by Mercury Intoxication. During an experiment conducted by Oliveira et al., 2006, 7.5 mg/kg Methyl mercuric chloride were administered daily for a period of 3 days in ovariectomized rats. This induced a substantial decrease in the Luteinizing hormone-releasing hormone (LHRH) content in the medial hypothalamus and plasma Luteinizing Hormone (LH) levels of the castrated rats, respectively (Oliveira et al., 2006).
However, this susceptibility can be lessened through estrogenic replacement which promotes a protective role of estradiol in the reproductive system. In fact, following treatment with estrogen in castrated female rats, there was a decline in the mercury content and the restoration of the LHRL levels in the medial hypothalamus and anterior pituitary gland (Oliveira et al., 2006).
Lead
The exposure to toxic level of lead is seen in multiple industries and research indicates that lead toxicity has effects on the neurological, renal, hematological but mainly the reproductive system (Khalaf et al., 2019). It appears to influence the hypothalamic-pituitary axis causing blunted responses of Thyroid stimulating hormone (TSH), Growth hormone (GH) and Follicle stimulating hormone (FSH)/Luteinizing hormone (LH) to Thyrotropin-releasing hormone (TRH), Growth hormone releasing hormone and GnRH respectively (Doumouchtsis et al., 2009).
In a research done by Rebecca z. Sokol, Carole e. Madding & Ronald s. Swerdloff for the Department of Medicine UCLA, on male Wistar rats using a control of 0.0% water, 0.1% lead acetate and 0.3% lead acetate, it was seen that with increasing levels of lead there was a significant drop in the intra-testicular sperm count. It was also noted that no compelling changes were seen in the luteinizing hormone in the serum but there was a significant drop in the follicle-stimulating hormone. At the same time a significant decrease in ventral prostate weight was observed. The failure to demonstrate elevated LH and FSH values in the face of markedly decreased serum testosterone and ventral prostate weight values suggests either a predominant mechanism of action of lead toxicity at the level of the hypothalamic-pituitary axis or a combined defect involving the gonad and hypothalamic-pituitary sites(Sokol,n.d.).
Data published recently talks about an increase in the level of GnRH mRNA at the hypothalamic level and an increased level of luteinizing hormone mRNA at pituitary level. This shows that lead alters the reproductive axis by interrupting with feedback mechanisms at the level of the hypothalamus and the pituitary gland. (Maffucci and Gore, 2009). It has been conjectured that lead inhibits the secretion of GnRH from the hypothalamus and LH from the pituitary, there is also a secondary disruption of these hormones at molecular level with lead’s competition with zinc regions at the estradiol receptor. It is to be noted that lead exposed animals adapt to the metal’s toxic effects on the reproductive axis maybe due to the changes in levels and stored hormone levels of GnRH and LH (Marques et al., 2000). As demonstrated in Figure 3, the hypothalamic-pituitary-testicles axis shows the negative and positive feedback loop.Lead also significantly reduces the ACTH-induced steroid production in cultured cells and studies have shown that exposure to lead causes a surge in corticosterone production (Rana, 2014).
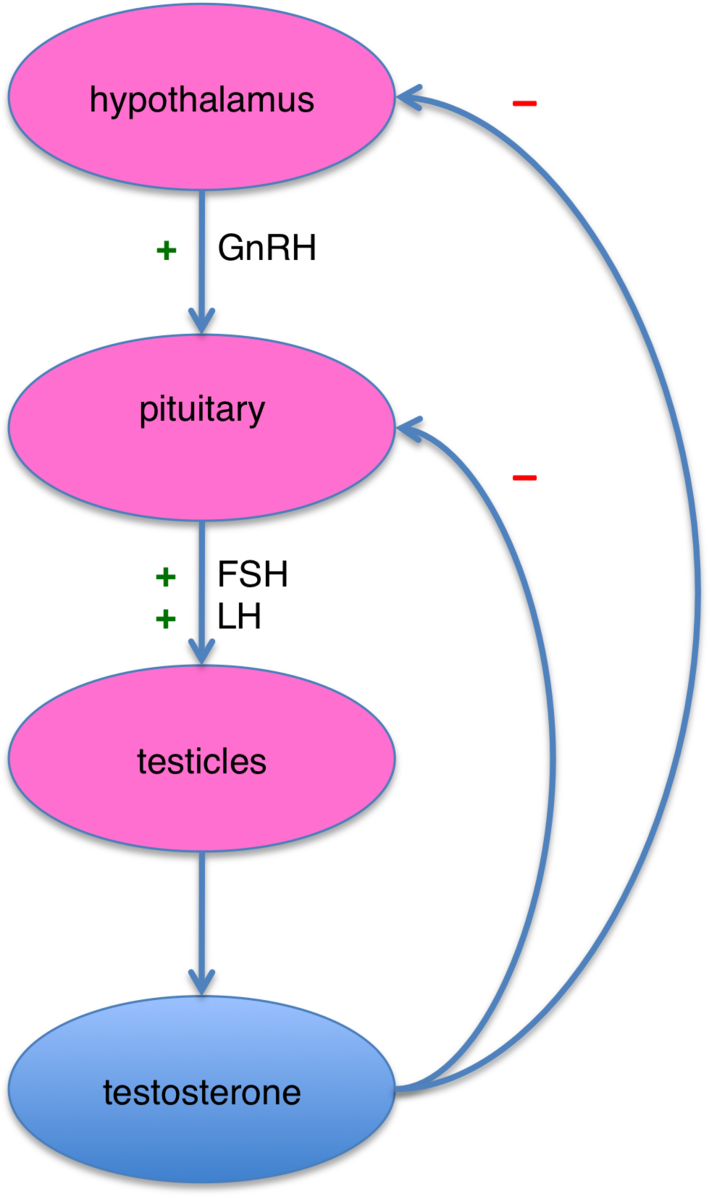
Figure 3. Hypothalamic-Pituitary-testicles axis
Conclusion
Toxic metals which are usually discarded as industrial or agricultural waste are consumed by humans via water or food chain causing adverse effects on the body, mainly on the CNS and the hypothalamic-pituitary-gonadal axis. Multiple studies have been conducted on rodents and it has been observed that the metals have an influence on the behavioural and cognitive functions as well as endocrine functions of the body by disruption of the mechanisms. It affects the chemical balance of catecholamines in the body, and the normal functioning of the reproductive system by modifying the levels of LH and FSH in the blood plasma. It has also been observed that the metals can affect the growth and induce weight loss. In case of maternal exposure to the toxic metals, harmful effects can be observed in the neonate. All these studies have shown that metal toxins affect humans and animals in various ways as the hypothalamus is the control centre for many bodily functions and have allowed the use of toxic metals to be more regulated by the industries.
References
Chan, A.;Webb, R.M.; Yang, C.M.; Jin, C.B. (1987): The Effect of Estrogen on Luteinizing Hormone-Releasing Hormone Binding Sites in Hypothalamic Membranes. Neuropharmacology 26:(9) 1395–1401
Clarkson, T.W. (2002): The Three Modern Faces of Mercury. Environ Health Perspect 110:11–231 Doumouchtsis, K.K.; Doumouchtsis, S.K.; Doumouchtsis, E.K.; Perrea, D.N. (2009): The Effect of Lead Intoxication on Endocrine functions. Journal of Endocrinological Investigation 32:175–183
Farina, M.; Aschner, M. (2019): Glutathione Antioxidant System and Methylmercury-Induced Neurotoxicity: An intriguing interplay. Biochim Biophys Acta Gen Subj. 1863: (12) 129285
Ferrer, B.; Peres, T.V.; Dos Santos, A.A.; Bornhorst, J.; Morcillo, P.; Gonçalves, C.L.; Aschner, M. (2018): Methylmercury Affects the Expression of Hypothalamic Neuropeptides That Control Body Weight in C57BL/6J Mice. Toxicological Sciences 163: (2) 557–568
Ferrer, B.; Prince, L.M.; Tinkov, A.A.; Santamaria, A.; Farina, M.; Rocha, J.B.; Bowman, A.B.; Aschner, M. (2021): Chronic Exposure to Methylmercury Enhances the Anorexigenic Effects of Leptin in C57BL/6J male mice. Food and Chemical Toxicology 147: 111924
Kalra, S.P.; Kalra, P.S. (1983): Neural Regulation of Luteinizing Hormone Secretion in the Rat. Endocrine Reviews 4:(4)311–351 Khalaf, M.A.M.; Younis, R.H.A.; El-Fakahany, H. (2019): Effect of Low-Level Environmental Lead Exposure on the Onset of Male Puberty. International Journal of Toxicology 38(3): 209–214
Lafuente, A. (2013): The Hypothalamic–Pituitary–Gonadal Axis is Target of Cadmium Toxicity. An update of recent studies and potential therapeutic approaches. Food and Chemical Toxicology 59: 395–404
Liao, J.; Yang, F.; Chen, H.; Yu, W.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; Pan, J.; Liang, Z.; Tang, Z. (2019): Effects of Copper on Oxidative Stress and Autophagy in Hypothalamus of Broilers. Ecotoxicology and Environmental Safety 185:109710
López, M.; Lage, R.; Saha, A.K.; Pérez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; Deoliveira, R.M.; Castañeda, T.; Datta, R.; Dong, J.Z.; Culler, M.; Sleeman, M.W.; Alvarez,C.V.; Gallego, R.; Lelliott, C.J.; Carling, D.; Tschöp, M.H.; Diéguez, C.; Vidal-Puig, A. (2008): Hypothalamic Fatty Acid Metabolism Mediates the Orexigenic Action of Ghrelin. Cell Metabolism 7:(5) 389–399
Maffucci, J.A.; Gore, A.C. (2009): Chapter 2 Hypothalamic Neural Systems Controlling the Female Reproductive Life Cycle: Gonadotropin‐Releasing Hormone, Glutamate, and GABA. International Review of Cell and Molecular Biology 274: 69–127
Meier, S.; Morton, H.C.; Andersson, E.; Geffen, A.J.;Taranger, G.L.; Larsen, M.; Petersen, M.; Djurhuus, R.; Klungsøyr, J.; Svardal, A. (2011): Low-Dose Exposure to Alkylphenols Adversely Affects the Sexual Development of Atlantic Cod (Gadus Morhua): Acceleration of the Onset of Puberty and Delayed Seasonal Gonad Development in Mature Female Cod. Aquatic Toxicology 105:(1-2) 136–150
Oliveira, F.R.T.; Ferreira, J.R.; Corrêa dos Santos, M.C.; Macêdo, L.E.M.;de Oliveira, R.B.; Rodrigues, J.A.; do Nascimento, J.L.M.; Faro, L.R.F.; Diniz, D.L.W.P. (2006): Estradiol Reduces Cumulative Mercury and Associated Disturbances in the Hypothalamus–Pituitary axis of Ovariectomized Rats. Ecotoxicology and Environmental Safety 63: (3) 488–493
Rana, S.V.S. (2014): Perspectives in Endocrine Toxicity of Heavy Metals-A Review. Biological Trace Element Research 160: 1–14 Romero, A.; Caride, A.; Pereiro, N.; Lafuente, A. (2011): Modulatory Effects of Melatonin on Cadmium-Induced Changes in Biogenic Amines in Rat Hypothalamus. Neurotoxicity Research 20: 240–249
Sokol, R.; Carole, M.; Swerdloff, R. (1985): Lead Toxicity and the Hypothalamic-Pituitary-Testicular Axis. Biology of reproduction 33:722-728
Tobwala, S.; Wang, H.J.; Carey, J.W.; Banks, W.A.; Ercal, N. (2014): Effects of Lead and Cadmium on Brain Endothelial Cell Survival, Monolayer Permeability, and Crucial Oxidative Stress. Markers in an in Vitro Model of the Blood-Brain Barrier. Toxics 2: 258-275
Zhang, Q.F.; Li, Y.W.; Liu, Z.H.; Chen, Q.L. (2016): Reproductive Toxicity of Inorganic Mercury Exposure in Adult Zebrafish: Histological Damage, Oxidative Stress, and Alterations of Sex Hormone and Gene expression in the Hypothalamic-Pituitary-Gonadal axis. Aquatic Toxicology 177: 417–424
Books
Klein, B.G. (2020): Cunningham’s Textbook of Veterinary Physiology, 6th ed. ELSEVIER. Blacksburg, VA
Marques, P.; Skorupskaite, K.; George, J.T.; Anderson, R.A. (2018): Physiology of GNRH and Gonadotropin Secretion
Figures
Figure 1: ‘Hypothalamus-pituitary’ (2019). Commons Wikimedia. Available at https://commons.wikimedia.org/wiki/File:Hypothalamus-Pituitary.jpg (Accessed: 22 April 2021)
Figure 2: ‘Hypothalamus-Pituitary-Gonadal axis in females’ (2014). Commons Wikimedia. Available at https://commons.wikimedia.org/wiki/File:Hypothalamic%E2%80%93pituitary%E2%80%93gonadal_axis_in_females.png (Accessed: 27 April 2021)
Figure 3: ‘Hypothalamus-pituitary-testicles axis’ (2010). Commons Wikimedia. Available at https://commons.wikimedia.org/wiki/File:Hypothalamus_pituitary_testicles_axis.png (Accessed: 27 April 2021)
