The Effect of Lysophosphatidic Acid on the Vascular System
Catherine Dempsey, Geraldine Collins and Gráinne Gallagher
Contents
Abstract
Lysophosphatidic acid is a phospholipid derivative that can act as a signalling molecule. It has the ability to stimulate cell proliferation and has been linked to cancer and cardiovascular diseases. LPA acts on many parts of the vascular system including platelets, monocytes, endothelial wall and smooth muscle cells In this essay we will discuss the role of LPA in relation to different areas surrounding the vascular system. The positive and negative effects of LPA can be seen through biological processes that occur in the body, angiogenesis and vasculogenesis, Atherosclerosis and atherothrombosis and G protein signalling.In relation to G protein signalling five mammalian cell-surface LPA receptors have been identified to date, which have been appointed LPA1-LPA5. LPA1 is the most common of the five receptors, with high mRNA levels in the brain, colon, small intestine, placenta, and heart, and to some extent expression is seen in the pancreas, ovary, and prostate. (Hecht et al, 1996).
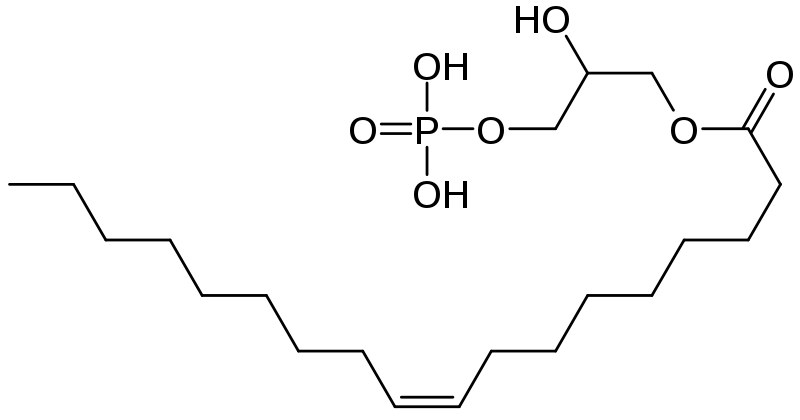
LPA is found in human plasma at a concentration of 0.4 – 0.5 µM 2 . According to Pages et al (2001), it is synthesized through a number of different pathways including de-acylation of phosphatidic acid(PA), by phospholipases A1 and A2 (PLA1 and PLA2), acylation of glycerol 3-phosphate, by glycerophosphate acetyltransferase, phosphorylation of monoacylglycerol (MAG), by acylglycerol kinase (AGK), and hydrolysis of plasma lysophosphatidylcholine (LPC), by the lyso-phospholipase D (lyso-PLD), autotaxin. 3
Figure 1
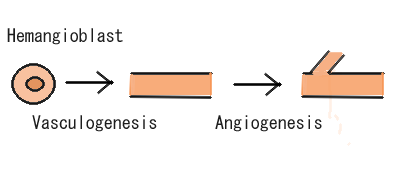
Angiogenesis
Research today shows there are many strong links between Lysophosphatidic Acid and its role in angiogenesis. Angiogenesis is the growth of blood vessels from the existing vasculature as seen in Figure 2. It occurs throughout life in both health and disease, beginning in-utero and continuing through to old age. No metabolically active tissue in the body is more than a few hundred micrometres from a capillary, the process of angiogenesis being responsible for this (Adair and Montani, 2010). Therefore, its role in vessel generation could be seen as one of the most important beneficial aspects of LPA on the vascular system.
Figure 2
Teo et al, (2009) used adult bovine aortic cells and human umbilical vein endothelial cells, to observe how LPA aided the closure of wounded endothelial monolayers of the endothelial cells in vitro. This was due to a stimulation of both endothelial cell migration and proliferation, brought about by LPA treatment. In another study by English et al (1999), LPA was shown to induce a chemotactic response in bovine pulmonary artery endothelial cells, similar in intensity to that observed with optimal levels of known endothelial cell chemo-attractants such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF). Possibly the most interesting case we found in the course of our research was an experiment carried out by Rivera-Lopez et al (2008), on the chicken chorioallantoic membrane (CAM) assay (a well-established system for gauging the angiogenic activity of small molecules). They found that LPA had a strong angiogenic effect in-vivo compared to the control, and the vessels generated were considerably more robust than the VEGF-treated CAM. Furthermore, they deduced that synthetic LPA receptor agonists and antagonists mimic, or block this response, respectively. (They also revealed three avian receptor types that are orthologous to the mammalian LPA(1), LPA(2), and LPA(3) receptors.)
As we know, angiogenesis is needed for normal embryo development, and for wound-healing, the beneficial angiogenic effects of LPA, but it is also a critical process for tumour growth. In order for effective tumour growth, the mass will attract new vessels to obtain its oxygen and nutrient supply. Teo et al (2009) reported that elevated LPA levels have been detected in the ascites of 98% of ovarian cancer patients, with 90% of patients with stage 1for early events in carcinogenesis. To add to the effects of LPA on the vascular system having adverse angiogenic effects aiding tumour growth, ovarian cancer cells have been found to produce LPA, thereby maintaining an LPA-rich environment. Using their results, they predicted that LPA receptor antagonists are a possible therapeutic route to inhibit angiogenesis in the case of tumour growth. Thus, we can deduce that although LPA has a positive effect on the vascular system with regard to angiogenesis, the angiogenesis stimulated may not always be beneficial, but instead having pathological effects.
Atherosclerosis
The effects of lysophosphatidic acid on the vascular system with an in-depth look at atherosclerosis. Atherosclerosis is the narrowing of the arteries due to the deposition of plaque. Atherosclerosis is a slowly progressing, multifocal, chronic arterial disease that is characterized by inflamma  tory and regenerative processes, which lead to matrix remodelling and large lipid deposits. The retention of low-density lipoproteins (LDL) and activation of endothelial cells initiate atherosclerotic lesion formation in the inner layer (intima) of medium and large-sized arteries, such as coronary arteries and cerebral arteries, predominantly at predilection sites, where the laminar blood flow is disturbed. As cardiac disease is a leading cause of death throughout the Western world, research into this area may prevent the influx in cardiovascular disease in further years to come.
tory and regenerative processes, which lead to matrix remodelling and large lipid deposits. The retention of low-density lipoproteins (LDL) and activation of endothelial cells initiate atherosclerotic lesion formation in the inner layer (intima) of medium and large-sized arteries, such as coronary arteries and cerebral arteries, predominantly at predilection sites, where the laminar blood flow is disturbed. As cardiac disease is a leading cause of death throughout the Western world, research into this area may prevent the influx in cardiovascular disease in further years to come.
LDL Deposition in Vessels
The correlation between LPA (lysophosphatidic acid) and Atherosclerosis can be seen through the description of Figure 3. Low density lipoproteins that have been modified by oxidation play an important role in atherosclerosis by increasing the amount of monocyte adhesion.
Figure 3
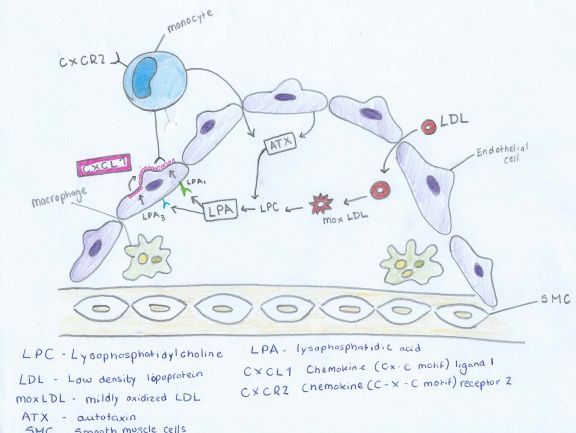
LDL (low density lipoprotein) enter thecells of the vessel wall. Here the LDL undergoes many modifications. Oxidation occurs. The degree of oxidation can occur in two different levels mildly oxidized LDL (mox - LDL) and extensively oxidized LDL (ox - LDL) (Stocker and Keaney, 2004)
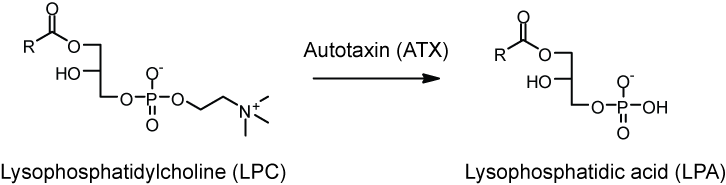
The mox-LDL increases the production of LPC (Lysophosphatidylcholine). The LPC is then converted into LPA by endothelial-derived ATX (autotaxin) into LPA as seen in Figure 4. LPA causes the release of the chemokine CXCL1 (chemokine ligand 1) from the endothelial cells when both the LPA1 and LPA3 receptors are activated. CXCL1 is released on the surface of the endothelial surface and this begins the process of monocytes binding to the vessel wall. The monocytes can then enter the subendothelial space and change into macrophages, which are the primary cells of atherosclerotic plaques.
Figure 4
Platelet activation and mild oxidation of LDL leads to LPA biosynthesis [(Siess et al, 1999) ( Aoki et al, 2008)], and elevated LPA levels are present in the atherosclerotic lesion core (Rother et al, 2003). We also found that LPA accumulated in and was the primary platelet-activating lipid of atherosclerotic plaques. Notably, the amount of LPA within the human carotid atherosclerotic lesion was highest in the lipid-rich core, the region most thrombogenic and most prone to rupture. Given the potent biological activity of LPA on platelets and on cells of the vessel wall, our study identifies LPA as an atherothrombogenic molecule and suggests a possible strategy to prevent and treat atherosclerosis and cardio cerebrovascular diseases (Siess et al, 1999). Through this repetitive cycle the plaque deposition increases and the width of the narrowing vessels decreases which in turn declines blood flow to the vital organs and back to the heart.
G protein signalling
As stated above, Lysophosphatidic Acid is a phospholipid derivative that can act as a signaling molecule. LPA and its sphingolipid homolog sphingosine 1-phosphate(S1P) and several other related molecules make up a family of lipo phosphoric acids that act as active receptor mediators. (Lynch et al, 2008) They have an important role in cell growth, differentiation, inflammation, immunomodulation, apoptosis and development. LPA and its related molecules act on a series of G protein-coupled receptors.
Many actions of LPA are mediated by G-protein coupled receptors. The trademark of G-protein receptors is their ability to identify and respond to chemically diverse ligands. LPA carry out their biological function by stimulating and activating G-protein coupled to a large family of cell surface receptors. 3 G-protein coupled receptors (GPCR’S) of the EDG (endothelial differentiation gene) family have been identified as LPA receptors; LPA1, LPA2, LPA3. (Lynch et al, 2008). There are also two other GPCR that are considered LPA receptors but these are distant to the EDG family and have not yet been studied in detail, LPA4 and LPA5. LPA may also act as an endogenous activator of PPARYgamma.
The set of GPRC’a that respond to LPA can activate the phosphatidylinositol 3 kinase (PI3-K)-AKT signaling pathway among others. LPA may potentially have a large impact on this pathway and therefore a large impact on cancer and other diseases.
As mentioned, LPA1-LPA3 are also known as Endothelial Differentiation, G protein coupled (EDG) family of LPA receptors. They are also known as this because they were first classified as orphan GPCRs involved in endothelial gene differentiation in human umbilical vein endothelial cells. (Riaz et al, 2016). In 1996, the study of Jerold Chun and his team lead to discovary of evidence to state that LPA is the ligand for EDG2. This was then followed by the discovery of LPA1, an S1P receptor and due to homology LPA3 and LPA4 were then discovered.
The importance of LPA1 has been examined and demonstrated through many different approaches and experiments. “In developing mouse fetus, Lpar1 is highly expressed in the nervous system. In knockout experiments, 50% Lpar1−/− mice experienced perinatal lethality and those that survived displayed retarded growth compared to the wild type mice”, (Riaz et al, 2016). LPA1 is present in all human tissues examined and is needed during development and adult life. LPA1 can activate members from three of four G-alpha subunit families which meads to downstream signaling. Downstream signaling pathways are important for the regulation of cell migration, cell multiplication, intracellular calcium mobilization and cell survival.
Receptor signalling
The testis and lymphocytes in humans express a high content of LPA2. The prostate, spleen, pancreas and the thymus show moderate content. Other important organs such as the brain and the heart don’t show any expression of LPA2. In mice, high levels of LPA2 are seen in the embryonic brain, kidney and testis, a more moderate content is seen in other organs. LPA2 mostly activates the same pathways as LPA1. There is one key attribute of LPA2, through connection with the thyroid receptor-interacting protein 6 (TRIP6) and stimulated by phosphorylation LPA2 can regulate cell migration. LPA2 promotes LPA-stimulated gastric cancer cells, in contrast to LPA1 stimulation of pancreatic cancer cells.
LPA3 shares sequence homology with LPA1 and LPA2. Adult humans express a high content of LPA3 in the heart, pancreas, prostate and the testis. Lower levels are found in the brain, lungs and ovary. High levels are found the heart and brain of an embryonic mouse. After birth the expression becomes more widely distributed.
LPA4, otherwise known as the non EDG LPA receptor, was discovered in 2003 by Kyoko Noguchi. This receptor shares no similarity with the EDG family of LPA receptors. Interestingly, LPA4 is the only receptor that activates adenyl cyclase and therefore increase the concentration of cAMP in cells.
LPA5, also known as a non EDG LPA receptor, was found to block migration and invasion of melanoma cells.
The most recently discovered LPA receptor is LPA6. So far it is known that this receptor is necessary for hair growth and it is also connected with RHO signaling.
Conclusion
During the course of our exploration into the subject of LPA and its vascular role, it has become clear to us that there are many research opportunities in this area, as it is an ongoing point of interest in medicine today. As we have highlighted in our essay, LPA has both beneficial and adverse effects on the vascular system. The investigation done by researchers thus far serves as valuable groundwork for future findings, that will no doubt aid our understanding of the relevant diseases, and hopefully open doors to preventative measures and possible cures to the pathological situations we have discussed. We have considered the vital role Lysophosphatidic Acid plays in angiogenesis, the subject we found to be the most beneficial aspect of LPA discovered to date. It is likely that an effective LPA receptor agonist/antagonist can be synthesised, to mimic or inhibit the angiogenic effects of LPA – the inhibitory possibility being a promising aspect in the control of tumour growth and invasion. On the other hand, we have researched the negative effects of LPA on the vascular system, its role in atherosclerosis as an atherothrombogenic molecule capable of oxidising LDL, being of interest to us. We have also reviewed the effect of LPA as a signalling molecule. The findings surrounding this area of our topic are young, and new discoveries are being made daily. We also can see the effects of G protein signalling in other areas of physiology such as neurophysiology and endocrinology which can be used as a reference to our understanding of the topic as a whole. We can see that LPA receptors can be found throughout the body and play a role in normal human response, such as inflammation and cell growth. On the other hand, adverse effect to health can also come into force in rapid cell growth, cancer. Here the knowledge surrounding LPA as a bioactive signalling molecule is crucial to our understanding and ability to treat cancer in both human and animals. It is clear that LPA plays a vital role in the physiological workings of the body both positive and negative, as described above, angiogenesis, atherosclerosis and G protein signalling.
References
Bibliography
Adair, T.H.; Montani, J.P. (2010): Angiogenesis. Colloquium Series on Integrated Systems Physiology: From Molecule to Function 2 (1): 1-84 http://www.ncbi.nlm.nih.gov/books/NBK53242/]]
Aoki, J.; Inoue, A.; Okudaira, S.; (2008); Two pathways for lysophosphatidic acid production. 1781 (9): 513-518. http://www.ncbi.nlm.nih.gov/pubmed/18621144
- Baker, D.L.; Desiderio, D.M.; Miller, D.D.; Tolley, B.; Tigyi, G.J. (2001): Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Analytical Biochemistry. 292: 287–295
- English, D.; Kovala, T.A.; Welch, Z.; Harvey, K.A.; Siddiqui, R.A.; Brindley, D.N.; Garcia, J.G.N. (1999): Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis 8 (6): 627-634.
- Hecht, J.H.; Weiner, J.A.; Post, S.R.; Chun, J. (1996): Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 135 (4): 1071–83.
- Pages, C.; Simon, M.F.; Valet, P.; Saulnier-Blache, J.S. (2001): Lysophosphatidic acid synthesis and release. Prostaglandins and Other Lipid Mediators. 64:1–10.
Riaz,A.; Huang,Y.; Johansson,S.; (2016):G-Protein-Coupled Lysophosphatidic Acid Receptors and Their Regulation of AKT Signaling. International Journal of Molecular Sciences. 17(2):1-68 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783947/
Rivera-Lopez, C.M.; Tucker,; A.L.; Lynch,; K.R. (2008): Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 11: (3) 301-310. http://[[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2677190/]]
Rivera-Lopez,C.M.;Tucker, A.L.; Lynch,K.R; (2009): Lysophosphatidic acid (LPA) and Angiogenesis. 11 (3): 301-310. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2677190/
Rother,E.; Brandl, R.; Baker, D.L.; Goyal, P.; Gebhard,H.; Tigyi,G.; Siess,W (2003): Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. 4: 741-747.http://www.ncbi.nlm.nih.gov/pubmed/12885756
Schober, A.; & Siess, W. (2012): Lysophosphatidic acid in atherosclerotic diseases. British Journal of Pharmacology, 167 (3): 465-482. http://www.ncbi.nlm.nih.gov/pubmed/22568609
Siess, W.; Zangl, K.J.; Essler, M.; Bauer, M.; Brandl, R.; Corrinth, C.; Bittman, R.; Tigyi, G.; Aepfelbacher, M. (1999): Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. 96 (12): 6931-6936. http://www.ncbi.nlm.nih.gov/pubmed/10359816
Stocker, R.; Keaney, J.F.(2004): Role of oxidative modifications in atherosclerosis. 4: 1381-478 http://www.ncbi.nlm.nih.gov/pubmed/15383655
Teo, S.T.; Yung, Y.C.; Herr, D.R.; Chun, J. (2009): Lysophosphatidic Acid (LPA) in Vascular Development and Disease. IUBMB LIFE 61 (8): 791-799 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4307796/]]
Image references
1.https://en.wikipedia.org/wiki/Lysophosphatidic_acid 2.en.wikipedia.org/wiki/Angiogenesis
