Stress related plasticity of the hypothalamus
Sarah Clarke, Ciara O’Sullivan, Johanna Rood
Introduction
Stress initiates an immediate response of multiple neural and endocrine systems (Bains et al, 2015). Both humans and animals respond to environmental anxiety with a stress response that allows for an adaption to the stressor to maintain homeostasis (Sheng et al, 2020). The plasticity of the hypothalamus is its ability to change and adapt to new information and stressors. Recent studies suggest that stressful experiences leave indelible marks on the paraventricular nucleus (PVN) of the hypothalamus and alter the ability of their synapses to undergo plasticity (Bains et al, 2015). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been related to a range of affective and stress related disorders (Levy and Tasker, 2012).
The hypothalamus and its plasticity
The hypothalamus is located within the diencephalon of the brain along with the thalamus and consists of several subnuclei. It has a major role in regulating the autonomic nervous system (ANS), subdivided into the parasympathetic (PNS) and sympathetic nervous system (SNS) (Klein, 2013). The hypothalamus secretes hormones and sends numerous neural impulses signalling the hypophysis, commonly known as the pituitary gland. The hypophysis has a major role in maintaining homeostasis of many biological processes, for example controlling the cardiovascular system, fluid distribution and thermoregulation (Klein, 2013). Neural and hormonal signals are delivered to target tissue by the SNS and PNS division. Nerve impulses are usually rapid and have a short duration of action, in contrast the endocrine system relies on chemical messengers that will produce a slower end result but with often long-lasting effects (Aspinall, 2003).
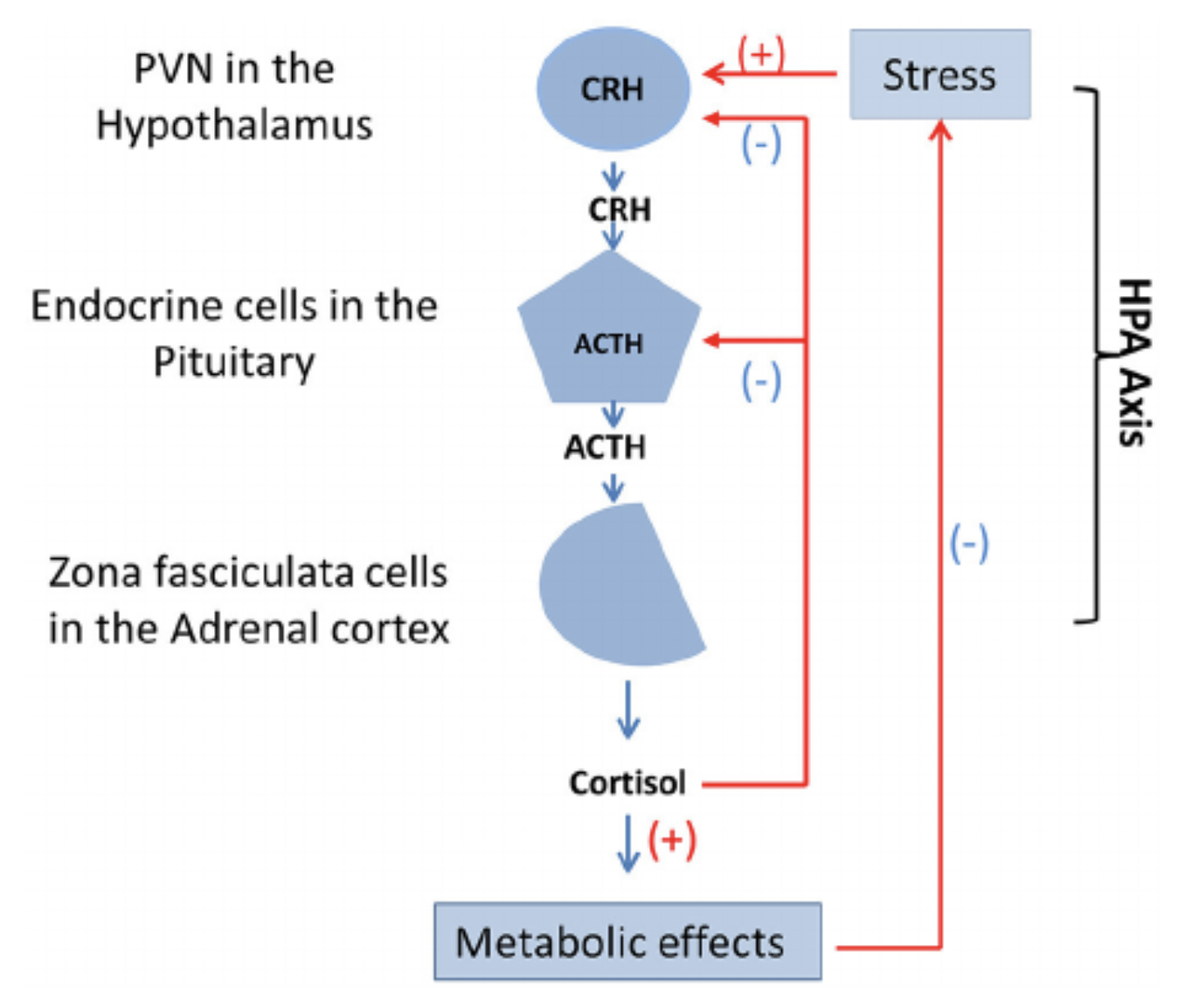
The HPA axis (figure 1) is one such neuroendocrine mechanisms that mediates the effects of stressors by regulating many physiological processes such as immune responses, metabolism and the ANS. The HPA axis conveys a cascade of endocrine pathways that respond to communication with the hypothalamus, pituitary- and adrenal glands via a negative feedback loop (Sheng et al, 2020).
|
Figure 1 The hypothalamus-pituitary-adrenal axis - (used under CC) |
Corticotropin-releasing factor (CRF) is responsible for activating the HPA axis as part of the common stress response pathway. The release of this hormone is initiated by the PVN of the hypothalamus as a response to relevant stress signals and works via neuroplasticity related mechanisms. Meng et al (2018) suggest this includes such responses as changing the amount of glutamate released and regulating the number of glutamatergic synapses on CRF neurons.
The hypothalamus retains forms of plasticity throughout life and immature synapses can frequently be found in the hypothalamus of the adult. The magnocellular system for example, shows plasticity during changes in water homeostasis. The hypothalamo-neurohypophysial system in the adult undergoes activity-dependant, reversible morphological changes which result in reduced astrocytic coverage of its neurons and an increase in their synaptic contacts (Dietrich and Hovath, 2005). In contrast, Theodosis et al, 2004 suggest that recent observations show that neurons and glial cells of the hypothalamo-neurohypophysial system continue to express ‘embryonic’ molecular features which may conclude their ability and capacity to undergo plasticity.
The studies of plasticity and predictive responses show that reprogramming occurs across almost all species. Morphological changes can occur due to a range of environmental cues, which can alter gene expression to help conform to recurring environmental pressures (DeWitt and Scheiner, 2004). Further research is needed to clearly determine how the plasticity of the hypothalamus is attained, however some stressors that can influence this have been established.
Stressors and the generalised stress response in the hypothalamus
Reser (2016) states that stressful situations are unpredictable and high-level cognition may be less effective during these times. In this case, the animal should increase its dependence on instinctual behaviours for survival that are controlled by the lower brain centres. Thus, confirming stress has an immediate effect on brain functionality and certain stressors will affect the brains morphology in many ways.
Marino et al (2019) state that the brain is the organ determining what is a non-life threatening and life-threatening stress. The brain further orchestrates the behavioural and physical responses which could cause health promoting or health damaging effects. The brain changes in its architecture, molecular profile and neurochemistry under acute and chronic stress, directing many body systems such as the cardiovascular and immune system leaving short and long-term consequences of being “stressed out” and potentially becoming health damaging.
Hormones released by the hypothalamus associated with chronic stress function to protect the body in the short term, promoting adaptation which should help overcome the stressor. However, if such a condition is not met the stressor will become permanent and the chronic stress response will initiate changes that may affect mood, memory, decision making and general health and wellbeing amongst others (McEwen, 2012).
It has been found that desensitisation to stressors on a long-term basis decreases secretion of peripheral HPA (hypothalamic-pituitary-adrenal) hormones such as ACTH (Armario et al, 2004). Researchers have also found sufficient evidence to support the hypothesis that long term exposure to a certain stressor can decrease the response of central parts of the hypothalamic-pituitary-adrenal axis such as gene expression in the PVN in the hypothalamus (Armario et al, 2004).
A review of studies by Lightman et al (2020) investigates if the stress response elicited by ACTH is dependent on a pulsating release of the hormone to prevent downregulation. ACTH will regulate the release of, amongst others, corticosterone from the adrenal gland. In rats where the adrenal gland had been removed, such animals receiving a constant exposure to corticosterone showed a significant impairment in the stress response compared to rats receiving corticosterone in physiological pulsatile doses. This confirms that even with part of the feedback mechanism removed, the hypothalamus can adapt to a certain degree but cannot always overcome certain stressors, i.e. pathological changes or unrelenting environmental conditions.
Other than the generalised stress response, often stressors have specialised pathways in the brain. For the purposes of determining plasticity of the hypothalamus however, the authors of this paper will only highlight individual stress responses relating to nutritional, heat and transport stressors. It should be noted that stressors are often interrelated and can start a cascade of biological responses which can affect many regulatory systems. For instance, a general stress response will immediately initiate a lack of hunger.
Nutritional stressors
A particularly important stressor among many species is the lack of available food and the following hunger or nutritional deficits. Metabolism and appetite-related processes are controlled by the arcuate nucleus in the hypothalamus (ARH) (Jenson et al, 2021). The interoceptive sensory neurons of the ARH are part of a greater neuronal network that measure certain factors in circulation such as glucose and hormones such as leptin and ghrelin that can indicate a change in the metabolic state (Langlet et al, 2013).
Corticotropin releasing hormone (CRH) initiates the hypothalamic-pituitary-adrenal response to stress. This can have an effect on food intake in both humans and rats leading to short- term anorexia. CRH acts as a neurotransmitter and activates CRHR1 and CRHR2 receptors. These types of receptors have a vital role in reducing food intake up to 48 hours as they have a short-term effect on the hypothalamus affecting the overall metabolism (Rabasa and Dickson, 2016).
Such a change in the metabolic state in experimental condition is often attained by fasting the animal or a specific diet withholding a particular foodstuff simulating certain nutritional deficiencies. Through fasting of mice Yang et al (2011) concluded that presynaptic glutamate release onto specific neurons is enhanced in such a state.
Further research found that leaving mice deprived of food overnight would induce a substantial rise in the expression of glial fibrillary acidic protein (GFAP) in the astrocytes of the ARH. It was also established that there was an increase in the mitochondria of astrocytes in food-deprived animals, whilst it was noted that the mitochondria appeared smaller in size (Varela et al, 2021). This research indicates that even short-term withholding of food can have an immediate effect on the plasticity of the hypothalamus. These findings are very current, having been completed in 2021. It should be noted however that the sample size was 15 cells from 4-5 mice and could be replicated on a larger scale to further validate these findings.
In summary, short-term plasticity of the hypothalamus is clearly linked to various pathways related to nutritional stress. Regardless of the adaptability of the hypothalamus, long term nutritional stress has severe health consequences including death.
Heat stress
It has been documented that there are many lingering effects of acute stress (Vitousek et al, 2018). Recent studies are now evidencing that stress can cause brain injury, induce neonatal death and memory loss in animals (Chauhan et al, 2017). Heat stress can cause morphological hypothalamic changes, oxidative stress and inflammation, it can also damage the structure and function of the nerve fibers in the hypothalamus (Slawinska et al, 2016). Heat stress can cause a disruption in the body’s normal physiological metabolism leading to a decrease in the ability to remove toxins causing oxidative stress. This triggers many pathways, nuclear factor erythroid 2 (Nrf2) is one of the most important signals in oxidative stress regulation.
Zhoa et al (2020) conducted research on the effects of heat stress on the hypothalamus in hens. In summary their research concluded that using dietary N-acetly-l-csyteine (NAC) can significantly treat and reduce heat stress hypothalamic injury by preventing factor Nrf2-related activation and controlling the Nrf2 pathway. It was concluded that NAC can help regulate the increase in blood glucose caused by heat stress and help to maintain a more stable blood glucose in hens. Understanding how organisms are affected by their environment and experiences has implications for a variety of biological disciplines including physiological changes, animal behaviour and community ecology and agriculture economics (Vitousek et al, 2018). Proving how essential ongoing research of long- and short-term effects of stress is vital for improving animal health and welfare and food science.
Transport stress
There is growing concern within communities about the treatment of farm animals including stress elicited by their transport and slaughter from an animal welfare as well as food quality point of view. To evaluate what kind of short-term stress effects can be expected, Hemsworth et al (2019) evaluated 399 lambs prior to transport on the farm with three behavioural tests taking into consideration a new environment, flight distance to a human and temperament as well as handling by stockperson and dogs. Blood samples were taken pre- and post-slaughter investigating plasma cortisol, lactate and glucose concentrations. The variables most indicative of stress and metabolic changes were negative handling behaviours of stockpersons such as fast movement and the physical handling of the lambs as well as aggressive behaviours of the dog. The study concludes that regression models indicating a combination of stockperson and dog behaviour on top of variables determined by the lamb behavioural test accounts for the most significant variance in plasma concentrations of plasma cortisol, glucose and lactate; respectively 33%, 34% and 44% pre- and post-slaughter. The authors highlight that these findings underline the relationship between handling and stress response, but that a direct causal link cannot be fully established.
A smaller study by Wang et al (2019) confirms physiological and psychological responses related to stress transport can also be measured in rats. Stress was simulated in a group of rats, which displayed behavioural changes after. Compared to rats not exposed to stress, increased corticosterone and norepinephrine concentrations were measured as well as a higher concentration of nitric oxide in the hypothalamus. Plasticity was established by looking at neuronal nitric oxide synthase (nNOS), which has a significantly increased expression particularly in the PVN. Whilst there was a clear increase in norepinephrine, cortisol and NO concentrations measured under stress conditions during transport, NO and nNOS proteins could be suggested as a more sensitive marker for measuring stress in animals.
This study shows a clearer causality compared to Hemsworth et al (2019), however it must be mentioned that Wang et al (2019) has a sample size of 12 rats only.
There is a wider range of stressors that can account for transport stress, such as capture and handling, unfamiliar sounds and vibration of transportation, collision, new group environments, unknown situations, temperature fluctuations, hunger and thirst (Wang et al, 2019) thus making it harder to directly establish causality between certain measurable physiological responses and the induced stress. However, it can be said for certain that regardless of what individual stressor is the cause, transport stress in general will induce a short term and measurable increase in NO, catecholamines and cortisol.
Long-term stressor-induced plasticity of the hypothalamus
Short term activation of the HPA axis is extremely adaptive due to the production of glucocorticoids causing an increase in gluconeogenesis, however this will suppress secondary physiological functions such as reproduction, the immune response and memory retention. In long term this effect will deplete the available glucose resulting in several physiological problems (Schell et al, 2013). Chronic stress can cause a great threat to the fitness of an organism by disrupting the normal HPA function (Schell et al, 2013).
Glutamate and GABAergic synapses in the PVN of the hypothalamus exhibit different forms of plasticity in response to stress. After acute stress these synapses can become conditionally excited due to the collapse of transmembrane chloride gradients and exhibit potentiation or depression. This extreme ability to adapt is due to its rich range of plasticity and is likely to represent an important building block for dynamic neuroendocrine stress adaptation (Bains et al, 2015).
The plasticity of the hypothalamus is induced by both stress and changes in glucocorticoid levels. The exposure to stress and fluctuations in circulating glucocorticoids results in pathological changes by evoking long lasting alterations in the parts of the brain regulating the HPA axis, including its plasticity (Levy and Tasker, 2012). GABAergic synaptic innervation of neurons located at the PVN undergoes significant plastic changes in response to acute stress. It was documented that in a 30–60-minute handling session of a rodent, the animal was under enough stress to cause a significant shift in the chloride gradient across the PVN, via the downregulation of the membrane potassium-chloride co-transporter. This shift in gradient causes post synaptic attenuation of inhibitory GABAergic inputs leading to the activation of the HPA axis (Hewitt et al, 2009).
Verkuyl et al (2005) discovered that acute stress seems to induce long-term presynaptic plasticity in the GABAergic inhibitory innervation of the PVN neurons via the transient elevation in circulating glucocorticoids. This research suggested that these presynaptic glucocorticoid actions are transcriptional and occur directly in the hypothalamus. Systemic glucocorticoid levels will also lead to long term shifts in the GABAergic synaptic innervation of the PVN neurons effecting the plasticity of the hypothalamus long term (Levy and Tasker, 2012)
Conclusion
Plasticity has been determined to be the ability of the brain to adapt to certain situations with physiological and phenotypical changes. Stress related factors contributing to such plasticity specifically in the hypothalamus were investigated. Research reviewed within this article can conclude that the hypothalamus exhibits extreme plasticity, both in the long and the short term. The brain is extremely versatile and adaptable to stressful situations, and its plasticity is often reversible in the short-term. However chronic stress will consequently lead to pathological changes including possible death of individuals in some cases. There are various markers that can be used to measure stress including ACTH, corticosterone, cortisol, glucose, lactate and norepinephrine. Recently measurement of nNOS has proven to be a more sensitive marker of stress and could strengthen plasticity theories in the future. It can also be concluded that measurement of plasticity is difficult due to the nature of the unknown ability of the brain, leaving open many areas of research in neuroendocrine complexes and its plasticity.
References
Armario A, Vallès A, Dal-Zotto S, Márquez C, Belda X (2004): A Single Exposure to Severe Stressors Causes Long-term Desensitisation of the Physiological Response to the Homotypic Stressor. Stress 7: (3) 157-172
Aspinall V (2003): Anatomy and Physiology of the Dog and Cat. The Endocrine System. Veterinary Nursing Journal 18: (6) 188-193
Bains JS, Wamsteeker Cusulin JI, Inoue W (2015): Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosc 16: (7) 377-88.
Chauhan NR, Kapoor M, Prabha Singh L, Gupta RK, Chand Meena R, Tulsawani R, Nanda S, Bala Singh S (2017): Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience 358: 79-92
Dietrich MO, Horvath TL (2005): Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends in Neurosciences 36: (2) 65-73
Hemsworth PH, Rice M, Borg S, Edwards LE, Ponampalam EN, Coleman GJ (2019): Relationships between handling, behaviour and stress in lambs at abbatoirs. Animal 13: (6) 1287-1296
Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS (2009): Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nature Neuroscience 12: 428-443
Jensen SB, Thodberg S, Parween S, Moses ME, Hansen CC, Thomsen J, Sletfjerding M, Knudsen C, Del Giudice R, Lund PM, Castaño PR, Bustamante YG, Velasquez MNR, Jørgensen FS, Pandey AV, Laursen T, Møller BL, Hatzakis NS (2021): Biased cytochrome P450-mediated metabolism via small-molecule ligands binding P450 oxidoreductase. Nat Commun 12: (2021) 2260
Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B (2013): Tanycytic VEGF-A Boosts Blood-Hypothalamus Barrier Plasticity and Access of Metabolic Signals to the Arcuate Nucleus in Response to Fasting. Cell Metabolism 17: 607-617
Levy BH, Tasker JG (2012): Synaptic regulation of the Hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neuroscience 6: (24)
Lightman S, Birnie M, Conway-Campbell B (2020): Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocrine Reviews 41: (10) 1210
Marino L, Rose NA, Visser IN, Rally H, Ferdowsian H, Slootsky V (2019): The harmful effect of captivity and chronic stress on the well-being of orcas (Orcinus orca). Journal of Veterinary Behaviour 35: (2020) 69-82
McEwen BS (2012): Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America 109: (2) 17180-17185.
Meng D, Li H, Deisseroth K, Leutgeb S, Spitzer N (2018): Neuronal activity regulates neurotransmitter switching in the adult brain following light-induced stress. Proceedings of the National Academy of Sciences of the United States of America 115: 5064-5071.
Rabasa C, Dickson SL (2016): Impact of stress on metabolism and energy balance. Current Opinion in Behavioral Sciences 9: 71-77
Reser JE (2016): Chronic stress, cortical plasticity and neuroecology. Behavioural Processes 129: 105-115
Schell C, Young J, Lonsdorf E, Santymire R (2013): Anthropogenic and physiologically induced stress responses in captive coyotes. Journal of Mammalogy 94: 1131-1140
Sheng JA, Bales NJ, Myers Sage A, Bautista AI, Roueinfar M, Hale TM, Handa RJ (2015): The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Frontiers in Behavioral Neuroscience 16: (7) 377-88
Slawinska A, Hsieh JC, Schmid CJ, Lamont SJ (2016): Heat stress and lipopolysaccharide stimulation of chicken macrophage-like cell line activates expression of distinct sets of genes. PloS One 11: (10)
Theodosis DT, Piet R, Poulain DA, Oliet SHR (2004): Neuronal, glial and synaptic remodeling in the adult hypothalamus: functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochemistry International 45: (4) 491-501
Varela L, Stutz B, Song JE, Kim JG, Liu ZW, Goa XB, Horvath TL (2021): Hunger-promoting AgRP neurons trigger an astrocyte-mediated feed-forward autoactivation loop in mice. J Clin Invest 131: (10) 144-239
Verkuyl JM, Karst H, Joëls M (2005): GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. European Journal of Neuroscience 21: (1) 113-121
Vitousek MN, Taff CC, Ardia DR, Stedman JM, Zimmer C, Salzman TC, Winkler DW (2018): The lingering impact of stress: brief acute glucocorticoud exposure has sustained, dose- dependent effects on reproduction. Proc R Soc B 285: (1882)
Wang J, Li J, Yu M, Wang Y, Ma Y (2019): An enhanced expression of the hypothalamic neuronal nitric oxide synthase in a rat model of simulated transport stress. MBC Vet Res 15: (2019) 323
Yang Y, Atasoy D, Su HH, Sternson SM (2011): Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146: (6) 992-1003
Zhoa Y, Zhuan Y, Shi Y, Xu Z, Zhou C, Guo L, Liu P, Wu C, Hu R, Hu G, Guo X, Xu L (2021): Effects of N-acetyl-l-cysteine on heat stres-induced oxidative stress and inflammation in the hypothalamus of hens. Journal of Thermal Biology 98
Other referenced materials
Klein BG (2013): Cunningham’s textbook of Veterinary Physiology, 5th edition. In: Introduction to the nervous system, pp 49-51. St. Louis, USA: Elsevier Saunders
DeWitt TJ, Scheiner SM (2004): Phenotypic plasticity: functional and conceptual approaches, pp 78-88. New York, USA: Oxford University Press
Image
Used under Creative Commons 4.0 International
Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, Niu R, Xie P (2017): Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Frontiers in Cellular and Infection Microbiology 7: (10)