Immunobiology of the Bovine Mammary Gland
Contents
Introduction
Mammary gland immunity, defined as the protection and resistance to infectious disease, is facilitated through a variety of immune and nonimmunological factors (Sordillo and Stretcher, 2002). The immune response is characterized by the ability to recognize the difference between foreign substances and the body’s own molecules. Upon recognition of invading pathogens, the immune system enlists both cellular and soluble factors that attempt to eliminate the foreign organism (Sordillo and Stretcher, 2002). In contrast, non- immune components of mammary gland immunity consist of a variety of disease-resistance mechanisms that are not specific to a particular pathogen and are not increased by repeated exposure to the same foreign molecule. The last 2 decades have seen major progress in understanding the bovine mammary gland defense system and its function in preventing disease (Sordillo and Stretcher, 2002).
The mammary gland is protected by a variety of defense mechanisms, which can be separated into two distinct categories: innate immunity and specific immunity (Gordillo et al, 1997).
Innate immunity, also known as nonspecific responsiveness, is vital for mammary gland health as it is the primary response during initial infection. At the site of infection nonspecific responses act quickly, after being triggered by various stimuli. However these responses do not increase with repeated exposure to the same infection or stimuli. Factors controlling nonspecific or innate responses of the mammary gland include: the physical barrier of the teat end, macrophages, neutrophils and by natural killer- like cells (Gordillo et al, 1997).
Acquired immunity, or specific immunity system, unlike innate immunity recognizes pathogens and once recognized eliminate these pathogens. Antibody molecules, macrophages, and several lymphoid populations all contribute to the recognition of various pathogenic factors. Repeated exposure to a pathogen can strengthen the immune response due to the “memory” trait of certain lymphocytes. Innate and acquired immunity play complementary roles in protection of the mammary gland providing optimal protection from disease (Gordillo et al, 1997).
Mastitis
Introduction
The production of immunoglobulins within the bovine mammary gland is very intensive due to the fact that the cow’s teat provides a channel for pathogens to enter the gland (Sordillo and Stretcher, 2002). Sordillo and Streicher (2002) state 'lactation is considered the final phase of the mammalian reproductive cycle, and the mammary gland provides milk for nourishment and disease resistance to the newborn'. In addition, lactation is the period where the mammary gland is most susceptible to environmental pathogens due to the expulsion of the mucous plug by the suckling of the newborn. The teat becomes exposed to airborne pathogens, pathogens transmitted from newborns, clostridial infections and (most importantly in dairy cows) the spread of pathogens from cow to cow during milking by the milker’s hands or the liner of the milking unit. Thus the role of immunoglobulins is of prime importance in the natural defence of the bovine mammary gland and the prevention of mastitis.
Role of Immune System in Mastitis
Mastitis is anything causing inflammation of the mammary gland, and infectious mastitis is caused by a plethora of microbes (Detilleu et al, 1995). Data from the National Mastitis Council from the 1990s suggest that mastitis affects one third of all dairy cows and will cost the dairy industry over 2 billion dollars annually in the United States in lost profits (Sordillo and Stretcher, 2002). Immunity against infectious diseases of cattle is mediated by diverse, yet co-dependent, cellular and humoral defence mechanisms. Many environmental and genetic factors (genetic predispositions etc.,) influence the ability of livestock to mount effective defence strategies against the various pathogens and normal flora that they are exposed to daily throughout their lifetime. Innate resistance to infectious diseases reflects the inherent physiological attributes of an animal that make it more or less susceptible to disease development by a particular pathogen (Detilleu et al, 1995). There are several cell lineages that comprise the immune system (e.g., B-cells, T-cells, neutrophils, eosinophils, basophils, macrophages and mast cells). Each of these cell types has distinct responsibilities in providing host defence. Innate or non-specific immunity represents the various immune components that are not intrinsically affected by previous contact with an infectious agent. The innate response is carried out by the physical barrier of the teat, neutrophils, natural killer-like cells and macrophages. Lymphocytes provide the adaptive immune reactions that are antigen specific in nature and possess memory for future encounters with the same pathogen (Kehrli, 2015). Antibodies/Immunoglobulins (Igs) are soluble glycoproteins that carry out the effector function for humoral and/or specific immune responses. Produced by antigen-activated B lymphocytes (which differentiate into Igs secreting plasma cells), immunoglobulins are either synthesised locally in lacteal secretions or are transported via serum.
Immunosuppression
Modern farming practices are placing a monumental amount of stress on dairy cows as producers seek to optimise milk yields and somatic cell counts. Studies have shown there is a negative correlation between milk production capacity and resistance to mastitis. A national survey carried out by ADHB Dairy found that, of 90 dairy herds across England and Wales almost a quarter of herds reporting greater than 100 cases per 100 cows per year (The UK Situation, 2009). In recent years, a vast amount of evidence of immunological dysfunction of lymhocytes and heterophils in pregnant cows has been generated by research institutes around the world. Periparturient immune dysregulation has a drastic impact on the prevalence of infectious diseases on essentially any organ system of livestock, according to Kehrli (2015). The cow’s immune system will become progressively more compromised at the end of gestation; which lasts between 280-290 days, and as a result the mammary gland will become more and more infected. Moreover, seasonal weather conditions will further the dampening effect on the immune system, as calving generally occurs in either Spring or Autumn, when temperatures are low, and forage quality is poor. Supplement to this, the fact that the majority of pregnant cows will be housed indoors in close proximity to one another during the winter months, which will dramatically increase the risk and speed of pathogens spreading from animal to animal. 1 or 2 weeks after calving the dam’s immune system will reach its lowest point and those subclinical infections begin to win the battle with the cow’s immune system and clinical mastitis results. Of the pathogens that cause clinical mastitis; E.coli, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus uberis are the most prominently encountered. Bacteriology results obtained from 480 clinical mastitis samples submitted for culture revealed Streptococcus uberis and Escherichia coli to be the most commonly isolated organisms, accounting for 23.5% and 19.8% of cases respectively in the UK (The UK Situation, 2009). A possible reason for the immunosuppression is the spike in levels of estrogen and progesterone having inhibitory effects on the function of immunglobulins. Many of the hormonal and metabolic changes that prepare the mammary gland for lactation take place during the 3 weeks preceding parturition (Comline et al, 1974). Lymphocyte and neutrophil function could possibly be affected by prepartal increases in estrogen, prolactin and growth hormone. Unfortunately the exact physiological reasons as to why this may occur have not been fully understood, but the evidence remains, and it is known that the periparturient immunosuppression reaches its optimum around 1-2 weeks after calving.
Colostrum
Introduction
Colostrum is a special milk secreted by the cow during the first two to three days after calving which is vital for the health of the calf. Colostrum in bovines is essential for transferring passive immunity to the newborn calf because no immunity is transferred prenatally via the placenta. Therefore calves are born with no immunity against disease. In the early part of life they depend entirely on the passive immunity acquired by drinking colostrum from their dam. Colostrum contains higher levels of nutrients and immunglobulins than regular milk, which play a critical role in the health of the newborn as an immune booster (He et al, 2001).
Transfer of Immunity
The importance of colostrum to newborn calves
The concentrations of protein and vitamins A, D and E in colostrum are about five times that of normal whole milk. The protein content of colostrum is 17–18% compared with 2.5–3.5% in regular milk. Newborn calves can only absorb immunoglobulins from colostrum through their intestine efficiently within the first 24 hours after birth. Therefore, it is very important that the calf either suckles naturally or is fed colostrum within this time frame in order to achieve the highest possible level of immunoglobulins (IgG) in the calf’s blood. Failure of passive transfer occurs when a calf has less than 10g of IgG per liter of blood when it is 24 to 36 hours of age. Research has shown a direct correlation between immunoglobulin levels in the calves' blood and the success rate of calves. Calves without adequate passive immunity are twice as likely to suffer disease and four times more likely to die, compared with calves that have an adequate level of passive immunity. For each hour delay of colostrum intake in the first 12 hours of life, the chances of a calf becoming ill increases by 10% (Moran, 2012). A delay by even 6 hours results in lower absorption of colostrum, which may lead to the calf becoming more susceptible to infections (Larson et al, 1980).
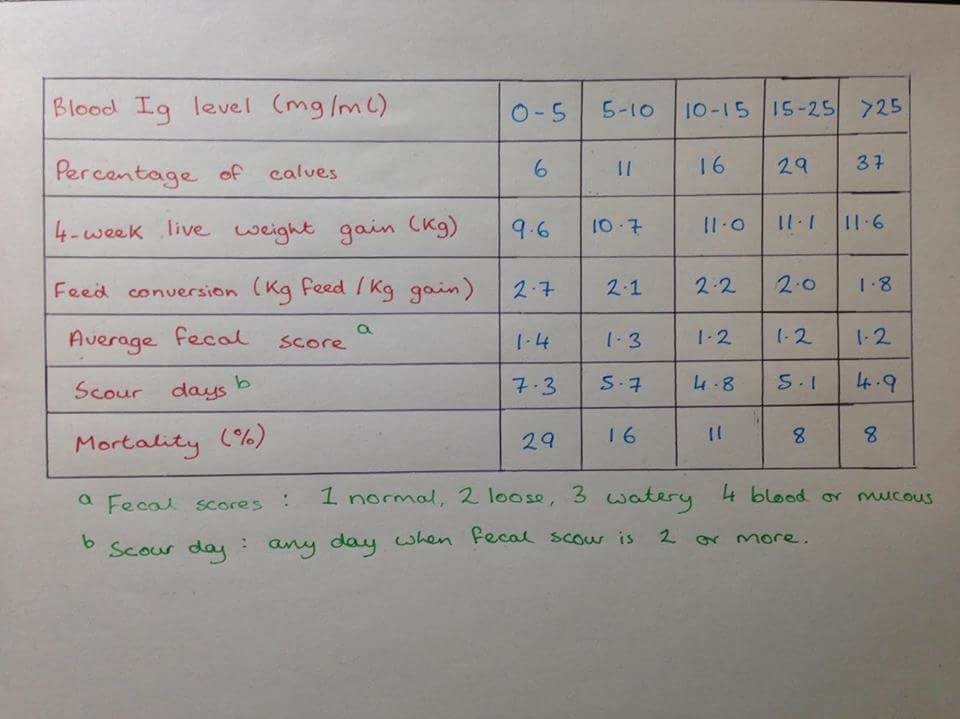
As we can see from the table below (Fig.1), as blood immunoglobulin levels increase calves' growth rates increase, their feed utilization becomes more efficient and their mortality rates decrease (Moran, 2012).
|
Other uses for bovine colostrum
After a few weeks the calves build up their own acquired immunity to different pathogens through direct exposure. The role of colostrum as an immune booster is not limited to the calf but can be administered to newborns of different species. Bovine colostrum is also widely used in infant humans and immunocompromised adults for the treatment and/ or prevention of enteric infections by bacterial, viral and protozoal pathogens (Tacket et al, 1988). In fact, consumption of raw cow's milk in the early life of a child was shown to be the most important factor in protecting against the predisposition to developing allergic hypersensitivity reactions (atropy) (Melnik et al, 2014) such as eczema and asthma.
Types of soluble defenses
Immunoglobulins in mammary secretions originate from both the bloodstream and the mammary glands themselves. Immunoglobulins in the mammary glands are produced locally by plasmacytes located adjacent to the secretory epithelium. Plasmocytes predominate in the involuted mammary gland and only occasional leukocytic cells are seen in mammary tissue sections taken during lactation (Larson et al, 1980). The epithelial cells in the bovine mammary gland are converted from a non-secretory state to a secretory state by a series of cellular changes called lactogenesis. Colostrum is produced during the first stage of lactogenesis in lactating cows. However colostrum is only secreted in the second stage of lactogenesis. The immunglobulins originating from the mammary glands are mainly in the IgA and IgM classes, while the immunoglobulins originating from the mother's blood are mainly IgGs (Larson et al, 1980).
Bovine colostrum typically contains between 50 – 150mg/ml of immunoglobulins. The approximate percentage of different immunoglobulin classes in bovine colostrum is as follows: IgG makes up roughly 85 to 90% of the immunoglobulins present (of which IgG1 accounts for 80 to 90%), IgM about 7%, and IgA about 5% (Larson et al, 1980). The mammary transport of immunoglobulins in the cow is highly selective. In contrast, the intestinal absorption phase in the calf is nonspecific for class of immunoglobulins and is operative essentially only during the first 24 h after birth under normal conditions (Larson et al, 1980).
Lactoferrin is a glycoprotein found in milk and other secretions such as tears and saliva. Lactoferrin is found at concentrations of aproximately 0.15g/L in bovine milk (Davies and South, 2015). Bovine lactoferrin (BLF) has a wide range of immune related and antimicrobial properties such as anti-inflammatory and anti-cancer effects, when ingested, for a number of different animal models (Yamauchi et al, 2006). It has been suggested that the protective effects of orally administered bovine lactoferrin are mediated by the immunomodulation of the intestine and systemic sites (Yamauchi et al, 2006). A short term increase in yield and concentration of lactoferrin in bovine milk can be achieved during lactation by extending the milking interval (Davies and South, 2015).
Cytokines are regulatory proteins that have roles in a number of different processes, such as tissue repair, inflammation and immunity, whereby they act as intercellular communication signals (https://www.abdserotec.com/cow-bovine-cytokines.html. ). Cytokines are grouped into early or late cytokines according to the timing of their involvement in the process of inflammation. Early cytokines include TNF-alpha, IL-1 and IL-6. These cytokines are produced by macrophages and stimulate the production of pro-inflammatory molecules by acting on various cell types at the site of infection (https://www.abdserotec.com/cow-bovine-cytokines.html. ). Late cytokines include IL-2, IL-4, IL-5 and IFN-gamma. The main role of these cytokines is to promote the differentiation and clonal expansion of specific immune cells (https://www.abdserotec.com/cow-bovine-cytokines.html. ).
The complement system has phlogistic, opsonic and bactericidal functions, which make the complement an important part of innate immunity. The milk of dairy cows with healthy mammary glands contains a low amount of complement due to different inhibitory mechanisms (Rainard, 2003). Once an inflammation develops, the complement components (which are derived from the blood) overcome the inhibitory mechanisms and the complement-dependent activities in milk will increase (Rainard, 2003). Important complement components in bovine mammary glands include C1q, C3b, C3bi and C5a.
Antigen Infusion
A number of studies have examined the area of infusion of antigen into the ruminant lactating gland (Larson et al, 1980). It has been found that antigen infusion often fails to elicit a local antibody response. However, infusion into the dry gland may result in a local IgA response that can persist into the following lactation. This response may be enhanced by simultaneous stimulation of the intestine (Larson et al, 1980). Some scientists have speculated that selecting cows for their high milk yield for many years may have led to alterations in the histology of the mammary gland which interferes with the establishment of IgA producing cells. This reasoning would help to explain the relative insensitivity of the bovine mammary gland to antigen infusion.
Conclusion
The mammary gland provides comprehensive protection against pathogens by means of both innate and acquired immunity. Nowadays however with an ever growing dairy industry and a huge emphasis on milk production (milk quotas rescinded in Ireland 2015) cows are becoming more and more susceptible to pathogen infections. With this we are seeing an increase in the amount of cases of mastitis worldwide. In contrast with greater understanding now in colostrum importance in newborn calve there has been a decrease in calf mortality in recent years. More and more farmers are looking into the immunobiological values of colostrum and are starting to make significant changes to the steps in calf welfare post birth. However such that the market they are in producers occasionally seek methods to improve the Ig content of colostrum, or look for alternatives to maternal colostrum.
The current generation of products designed to supplement or replace colostrum have utility in increasing circulating IgG concentration, but much more research is needed to more completely understand the role of non-Ig components of colostrum and their effects on long-term animal production and welfare (Hammer et al, 2004).
References
Comline R.S. , Hall L.W. , Lavelle R.B. , Nathanielsz P.W. , Silver M. (1974). Parturition in the cow: Endocrine changes in animals with chronically implanted catheters in the Fetal and Maternal Circulations. Journal of Endocrinology. 63 (3), 451-472.
Davies S. R., South, C. R.. (2015). Suspension of milking in dairy cows produces a transient increase in milk lactoferrin concentration and yield after resumption of milking. Journal of Dairy Science. 98 (11), 7823–7830.
Detilleu JC, Kehrli ME Jr, Stabel JR, Freeman AE, Kelley DH.. (1995). Study of immunological dysfunction in periparturient Holstein cattle selected for high and average milk production.. Veterinary Immunology and immunopathology. 44 (3-4), 251-267.
Gordillo L.M., Shafer-Weaver K, DeRosa D. (1997). Immunobiology of the mammary gland. Journal of Dairy Science. 80 (8), 1851-1865.
Hammer C.J. , Quigley J.D. ,Ribeiro L. , Tyler H.D.. (2004). Characterization of a Colostrum Replacer and a Colostrum SupplementContaining IgG Concentrate and Growth Factors. Journal of Dairy Science. 87 (1), 106-111.
He F. , Tuomola E., Arvilommi H. ,Salminen S. (2001). Modulation of human humoral immune response through orally administered bovine colostrum. FEMS Immunology & Medical Microbiology. 31 (2), 93-96.
Kehrli Jr, M.E., 2015, January. Immunological dysfunction in periparturient cows: Evidence, causes and ramifications. In 2015 Florida Ruminant Nutrition Symposium (p. 14).
Larson B.L. , Heary Jr. H.L., Devery J.E.. (1980). Immunoglobulin Production and Transport by the Mammary Gland. Journal of Dairy Science. 63 (4), 665-671.
Melnik,C.B. Swen Malte, J. Schmitz, G.. (2014). Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy. Journal of Translational Medicine. 12 (43), 3-10.
Moran.J (2012). Rearing Young Stock on Tropical Dairy Farms in Asia. Australia: CSIRO Publishing. 41-56.
Rainard P. (2003). The complement in milk and defense of the bovine mammary gland against infections. Veterinary Research. 34 (5), 647-670.
Recombinant Bovine Cytokines (2016). Available: https://www.abdserotec.com/cow-bovine-cytokines.html. Last accessed 5th May 2016.
Sordillo L.M., Stretcher K.L. (2002). Mammary Gland Immunity and Mastitis Susceptibility. Journal of Mammary Gland Biology and Neoplasia. 7 (2), 135-146.
Tacket C. O., Losonsky G., Link H. (1988): Protection by milk Immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. New England Journal of Medicine 318 (1), 1240-1243.
The UK Situation (2009). Available: http://www.mastitiscontrolplan.co.uk/the-uk-situation. Last accessed 21st Apr 2016.
Yamauchi K, Wakabayashi H, Shin K, Takase M. (2006). Bovine lactoferrin: benefits and mechanism of action against infections. Biochemistry and cell biology. 84 (3), 291-296.