The role of herpesviruses in the development of Alzheimer's disease
Contents
- Introduction
- Correlation between herpesvirus and Alzheimer disease
- How can we protect ourselves from Alzheimer disease?
- References and external sources
- Other external sources
Introduction
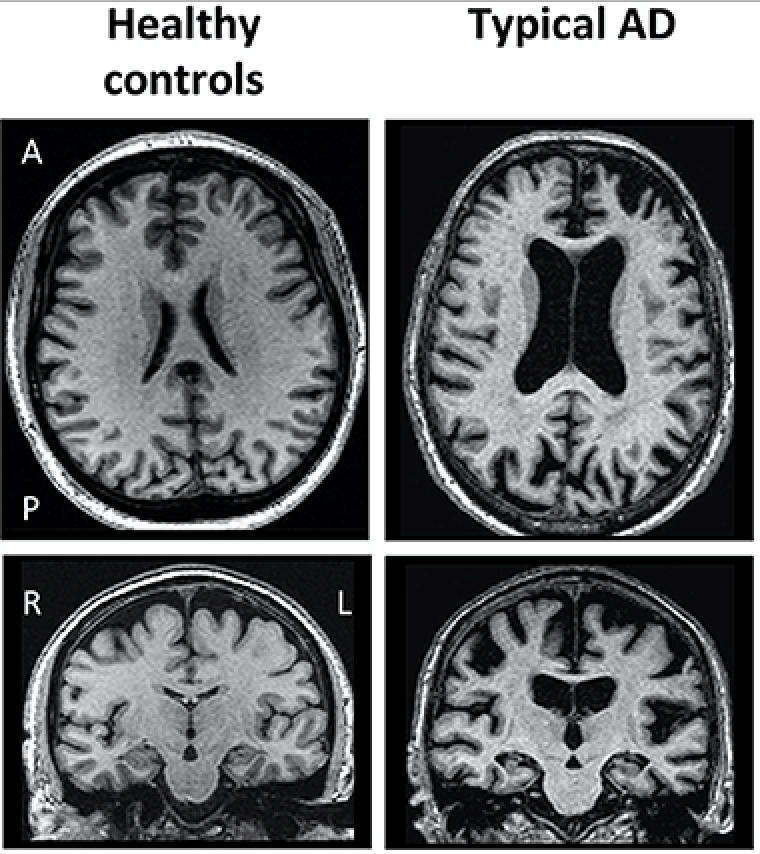
In this essay, the correlation between the Herpes virus and Alzheimer disease is discussed. Alzheimer disease was first described by the German psychiatrist and neuropathologist, "Alois Alzheimer" in 1906. Since then, studies have been investigated the disease and its origin. Nowadays, it is known that intercellular depositions of amyloid β (Aβ) protein (protein integrated into the neural membrane) result in plaques, which can accumulate in the brain tissue disrupting the neural signaling, causing inflammation and damage the blood vessels within the brain (Minter et al, 2015). In addition, it was found that Tau protein, an intracellular component necessary for the stability of the neuron cytoskeleton and normal synaptic activity, can form neurofibrillary tangles which are another major cause of apoptosis of the neurons (Maltsev et al, 2013). All these altered processes lead to the degenerative processes seen in the brain of Alzheimer patients (Figure 1). Different researchers have been trying to find the underlying cause for the impaired processes. While certain study groups offered genetic background as a possible cause, nowadays an increasing interest in the correlation between the herpes virus and Alzheimer disease is taken into consideration. The current findings associate two main types of herpes virus with the disease.
|
Figure 1 Brain scan of healthy brain VS. AD patient (Ferreira et al, 2019) |
Correlation between herpesvirus and Alzheimer disease
Herpesvirus types associated with AD
Since the discovery of Alzheimer disease, different studies have been trying to find a connection between certain types of herpes virus and the disorder. In most of the cases investigated, herpes simplex virus type 1 (HSV-1) was strongly correlated with the degenerative processes caused by the disease (Bourgade et al, 2014). However, recent studies have been claiming that Human Herpes Virus type 6 (HHV-6) may be associated with the disease as well (Readhead et al, 2018).
Physiological background
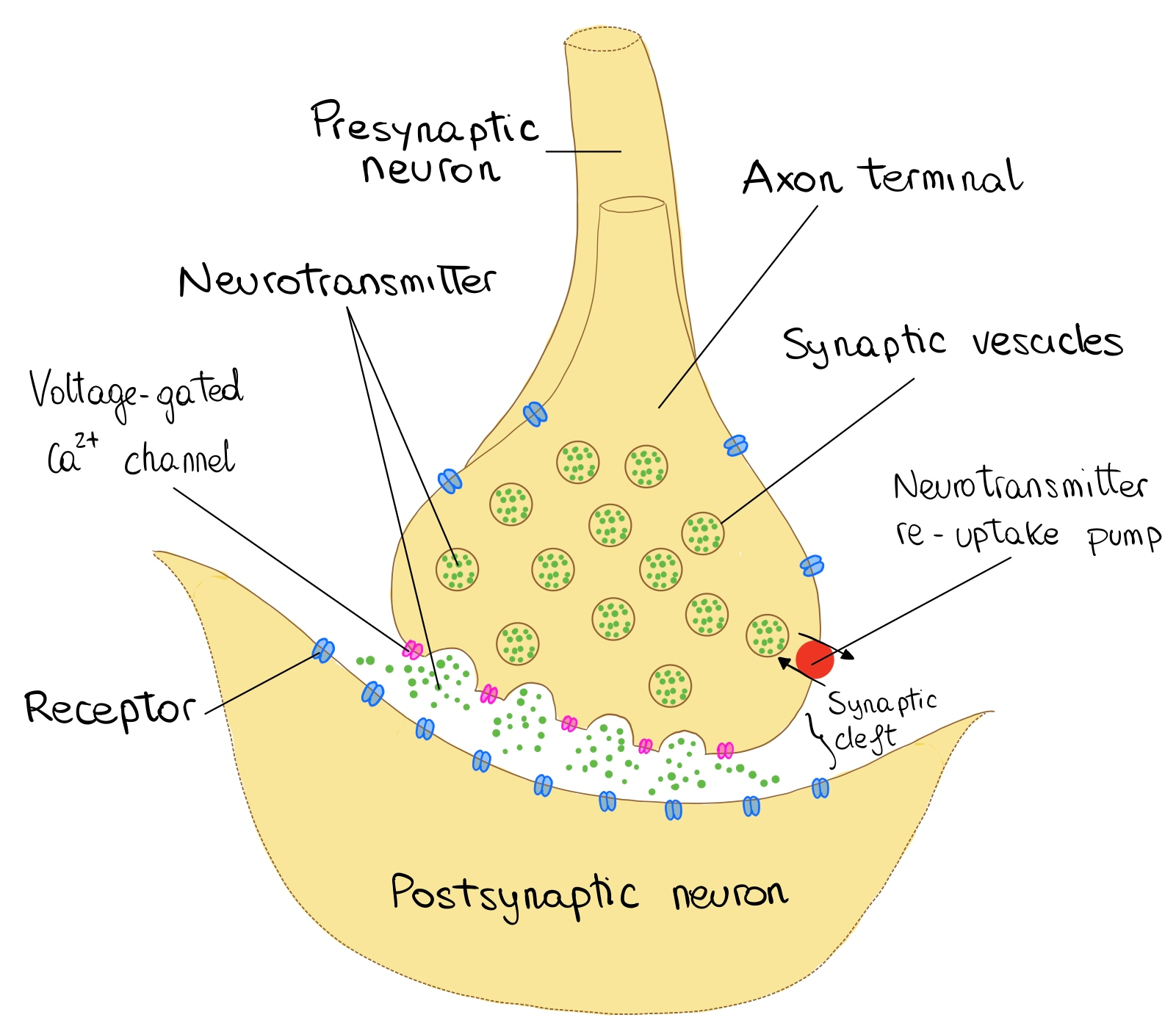
The basic functional unit of the nervous system is the neuron. Neurons are found within the central nervous system (the brain and the spinal cord), as well as in the periphery. Generally, the information is transferred from the presynaptic cleft of one neuron to the postsynaptic cleft of the other, via chemicals named neurotransmitters. A stimulus reaches the neural membrane depolarizes it locally. If the depolarization reaches the threshold potential, an action potential is generated in the cell soma (body). Then it continues along the axon until it arrives at the axon terminal, in which neurotransmitters are stored in vesicles. Via exocytosis, the neurotransmitters are released from the presynaptic cleft to the synaptic cleft (the electrical signal is transferred to a chemical signal). The released neurotransmitters bind to receptors found on the postsynaptic dendritic spines and by corresponding processes involving other ions another AP is generated. Thus, the signal is transferred to the adjacent neurons (Bae and Kim, 2017). Representation of neural communication can be seen in (Figure 2).
Neurodegenerative diseases as Alzheimer disease alter the structure of the neurons and the connections between them, by reducing the numbers of vesicles and proteins necessary for neural transmission. This leads to degradation of the neurons themselves as well as the neurotransmitters and damages the Ca2+ regulation, which are necessary for the normal functioning of the nervous system (Reith, 2018).
|
Figure 2 Communication between neurons |
(modified from communication between neurons by OpenStax College, Anatomy & Physiology, CC BY 3.0) |
Pathophysiology
Mancuso et al, (2019) investigated brain sections of Alzheimer patients, in which traces of herpes virus types mentioned above were found. In addition, the brain sections studied presented accumulations associated with amyloid β plaques outside the cells, and neurofibrillary tangles (resulted from Tau protein fragments) inside the cells. These findings were correlated with Apolipoprotein activity as well (ApoE4, a glycoprotein related to lipid transport between cells), suggesting that there is a relationship between the herpes virus and the degenerative processes linked to Alzheimer disease.
HSV-1 and amyloid B (antimicrobial process)
Amyloid Precursor Protein (APP) is a protein integrated into the neurons' membrane. It is essential for the normal development of the cell, regulation of the presynaptic active zone (O'Brien and Wong, 2011), as well as the synaptic plasticity and cell adhesion (Mancuso et al, 2019). In a healthy brain, APP is used, degenerates and recycled by beta and gamma-secretase enzymes, which cleave soluble parts of the protein. In Alzheimer patients with HSV-1, lower levels of APP and increased activity of gamma-secretase enzyme were found (Mancuso et al, 2019). These were associated with the cleavage of non-soluble fragments named Amyloid β, which aggregated and formed clusters, the amyloid β plaques. The plaques, which are first generated in the cortex (Oddo et al,2003), were shown to disrupt the neural signaling leading to the damages observed in the brains of Alzheimer patients.
HSV-1 and TAU proteins (deposition)
Tau protein is integrated into the microtubule system, which is a part of the neurons' cytoskeleton structure. It was found essential for the adequate transmission of information and normal neural activity and stability. Studies correlated HSV-1 with hyperphosphorylation of Tau protein in the hippocampal region (Powell-Doherty et al, 2020). The hyperphosphorylation was followed by a change in its structure and detachment from the microtubule system. The tau protein fragments then aggregated in paired helical filaments and formed tangles inside the cell. This resulted in alternations in the microtubules and cytoskeleton dynamics, which contribute to the dysfunction of neural communication activity and finally results in cell death (Harris and Harris, 2018).
Inflammation (suppression of immune system)
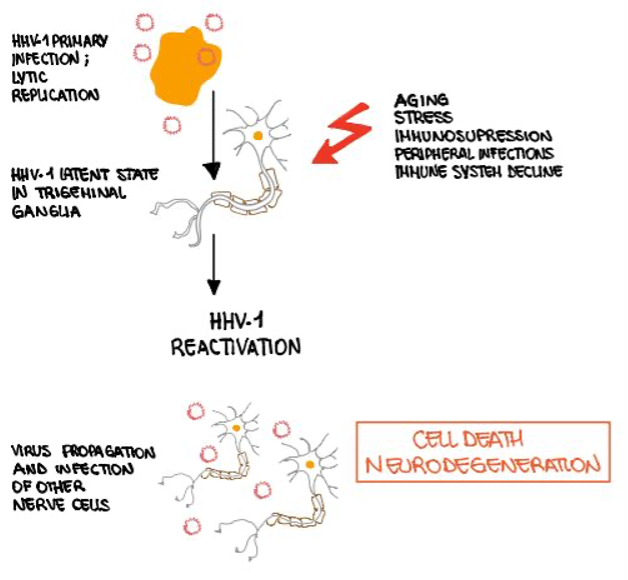
Sochocka et al (2017) correlated the neuroinflammatory responses to viral infections, particularly HSV-1 HSV-2, in Alzheimer patients (Figure 3). Following HSV-1 infection, increased activity of microglia and oligodendrocytes (glial cells which are associated with the immune defense of the CNS) was observed. In addition, it was suggested that the amyloid β plaques' appearance triggers the activation of anti-inflammatory processes and cytokines dysregulation which contributes to the progression of the disease. Moreover, the elderly population was found more susceptible to be infected by the virus due to suppressed immunity, contributing to higher permeability of the blood-brain barrier, which puts them at a higher risk to develop Alzheimer.
|
Figure 3 Schematic diagram – the effect of HS1 on neurons |
HSV-1 and oxidative stress
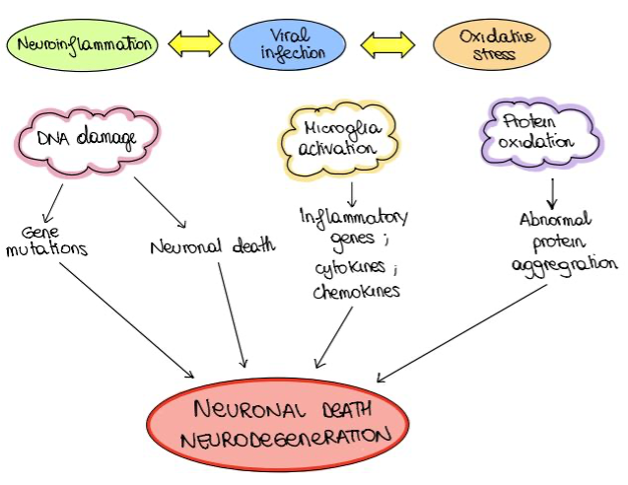
Oxidative stress (OS) occurs when there is an imbalance between antioxidants and free radicals within the cell. Limongi and Baldelli (2016) found that many viral infections, including HSV-1, induce oxidative stress associated with inflammation and neural degeneration (Figure 4). Oxidative stress correlated with HSV-1 was found to inhibit the secretion of amyloid β, resulting in the accumulation of amyloid β inside the cells. Moreover, OS and HHV-1 were suggested to impair the lysosomal activity within the cell, leading to the accumulation of lysosomal particles and finally cell degeneration (Kristen et al, 2018). Lastly, OS associated with HSV-1 was found to enhance mitochondrial damage, which can contribute to oxidative stress and neural degeneration (Harris and Harris, 2018). Thus, HSV-1 was proven to cause oxidative stress, which is suggested to increase the neurodegenerative processes linked to Alzheimer disease.
|
Figure 4 The relation between inflammation and oxidative stress |
HSV-1, HHV-6- and autophagy (autophagosome accumulation)
In healthy cells, autophagic activity is referred to the ability of the cell to induce intracellular immune activity. Autophagy is a process that involves several steps, in which the cell proteins are able to produce vesicles containing the detected viral particles. Via fusion of the virus-containing vesicles with cell lysosomes, the cell is capable of removing the harmful substances as well as misfolded and aggregated proteins and damaged organelles, which are nonfunctional cell particles (Romeo et al, 2019). Orvedahl et al (2007) associated HSV-1 and HHV-6 with impaired autophagic activity in Alzheimer patients. In addition, Romeo et al (2019) stated that the viral protein ICP34.5 of HHV-1 was found to inhibit Beclin-1, a phagocytic protein associated with the regulation of the cell phagocytic activity. HHV-6 was also associated with damaged autophagic activity, however, its mechanism of action is still under research. The impaired autophagic activity results in neurodegenerative processes and cell death seen in Alzheimer patients. Herpes virus types that were linked to these neurodegenerative processes, were suggested to contribute to these processes via the dysregulation of autosomes, accumulation of Amyloid β plaques, and intracellular fibrillary tangles (Orvedahl et al, 2007).
HSV-1 and apolipoprotein
ApoE is a gene responsible for the expression of apolipoproteins, which are glycoproteins necessary for the transport of different lipid molecules between tissues. ApoE has 3 isoforms (ApoE2, 3 and 4), which differ from each other in their nucleic acid sequence. Alzheimer patients with HSV-1 traces were associated with the ApoE4 allele, which is suggested to make them more vulnerable to HSV-1 infection. In order to enter the cell, HSV-1 binds to the HSPG membrane receptors. ApoE competes with HSV-1 for these binding sites, while ApoE4 was found to be less efficient than its other isoforms leading to the entrance of the virus particles and cell infection (Harris and Harris, 2015). Moreover, ApoE4 was correlated with neuroinflammation and proinflammatory factors, alternation in synaptic plasticity, lipid metabolism, cell signaling and increased permeability of the blood-brain barrier. All contribute to the progression of the disease (Harris and Harris, 2015).
Treatment (Future research) - Antiviral medication
Valacyclovir
Valacyclovir is the most widely used antiviral drug against HSV-1 and HSV-2, which can pass through the blood-brain barrier. As HSV-1 and HSV-2 were suggested to increase the susceptibility for Alzheimer, new trials suggest that the drug may be effective against the disease as well. Valacyclovir is the pro-drug of acyclovir which can inhibit the viral polymerase activity, leading to the death of the infected cells but not healthy ones (Devanand, 2018). Further findings show that administration of valacyclovir and acyclovir reduces the accumulation of amyloid β extracellularly and decreases the phosphorylation of Tau protein (and subsequently the formation of the tangles) associated with Alzheimer disease. The Valacyclovir was approved by the FDA (Food and Drug Administration) as a treatment in the therapy of Alzheimer patients (Harris and Harris, 2015).
VZV vaccine
VZV (Varicella-Zoster virus) vaccine is administered as part of the treatment given to patients detected with herpes. High doses of the vaccine resulted in a decrease in the activity of the virus and strengthen the immune system (by increasing the antibody production) in elderly patients between 50-59 years (Qin and Li, 2019). Therefore, it was suggested that administration of VZV may lower the susceptibility to Alzheimer disease.
Can Alzheimer develop in the absence of HSV-1?
Different studies have shown that other risk factors are involved in the development of Alzheimer disease. Genetic background is one of the causes of allelic mutations on chromosome 1, 14 or 21, which were linked to amyloid β plaques formation. In addition, another mutation in the ApoE gene was found to contribute to the progression of the disease as well (Hoyer, 2000). Stress, depression and inadequate sleep were shown to affect the hypothalamic-pituitary-adrenal axis (HPA axis), leading to increased cortisol production found to increase the amyloid β depositions in the hypothalamus and prefrontal cortex. Other studies linked the ApoE mutation with the increased cortisol production and consequently the development of Alzheimer disease(Silvia et al, 2019). Smoking is another factor that may affect the development of Alzheimer disease, as it generates the formation of free radicals, increases oxidative stress and promotes proinflammatory activity. In addition, it can lead to cerebrovascular diseases which increase the likelihood of the appearance of the disease (Silvia et al, 2019).
How can we protect ourselves from Alzheimer disease?
Unfortunately, no cure for Alzheimer disease has been found. However, there are recommended lifestyle behaviors, which may decrease the possibility to develop the disease. Physical activity was suggested to decrease the risk for developing Alzheimer by 45% (Silvia et al, 2019). It was correlated with reduced blood pressure, lower risk to develop obesity, strong proinflammatory activity, better cerebral blood flow, more efficient oxygenation of the brain tissue, as well as the increase of IGF-1 (insulin-like growth factor), BDNF (brain-derived neuropathic factors) and VEGF (vascular endothelial growth factor). All were found to induce synaptic plasticity and synthesis of neural factors necessary for the normal function of the nervous system. Mediterranean diet with its many beneficial health constituents such as unsaturated fat (found in olive oil or fish oil), antioxidants, vitamins E, C, B6 and B12, was also suggested to decrease the susceptibility to develop Alzheimer disease. In addition, vitamin D deficiency was correlated with an increase in the amyloid β depositions and a decrease in their degradation (Silvia et al, 2019), pointing out the importance of adequate vitamin D consumption for the normal function of the nervous system.
References and external sources
Other external sources
Figure 2: Communication between neurons